Table of Contents
ToggleWagner meerwein rearrangement reaction, examples, mechanism, and application have been discussed here. This reaction was first of all reported by Wagner in 1899 and further extended by Meerwein in 1914.
wagner meerwein rearrangement reaction
Wagner meerwein rearrangement is an acid-catalyzed rearrangement of alcohol in which the alkyl group migrates to give more substituted alkenes. This reaction is also called Wagner meerwein shift or wager meerwein migration reaction.
- This rearrangement is 1,2-rearrangement of carbocation from which the migratory group such as phenyl,vinyl allyl or hydrogen etc. migrates from adjacent carbon to carbocation carbon to get more stable carbocation.
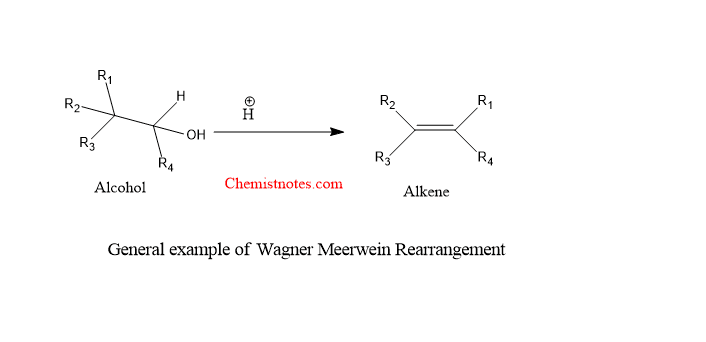
wagner meerwein rearrangement example
Let’s see some examples of Wagner meerwein rearrangement reaction.
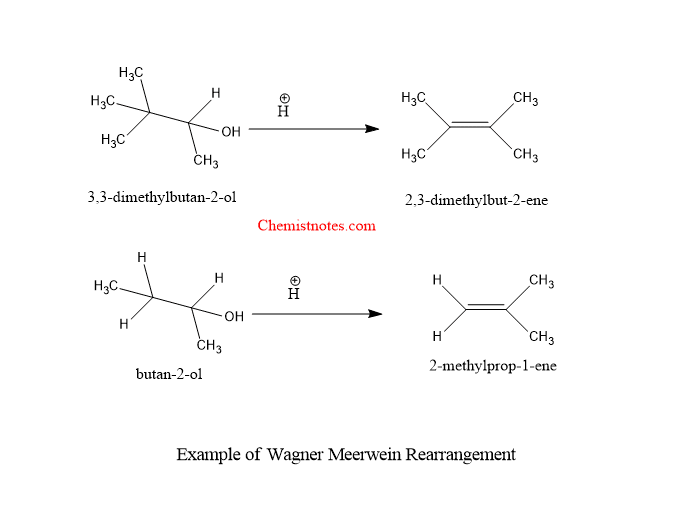
wagner meerwein rearrangement mechanism
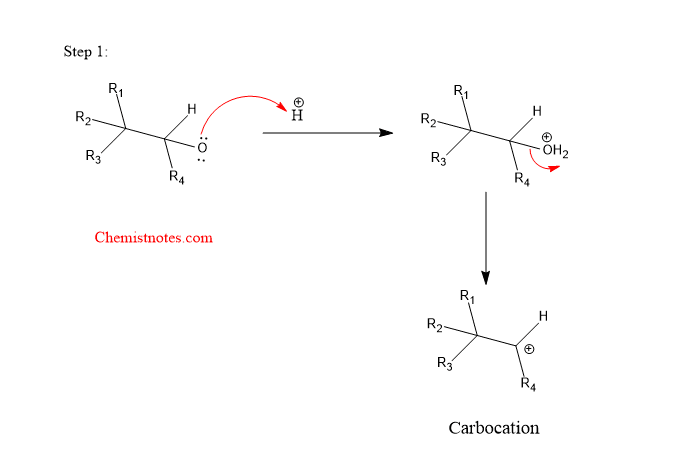
The Wagner meerwein rearrangement reaction is a step reaction that completes in the following two steps:
Step 1: Protonation of alcohol followed by the formation of a carbocation.
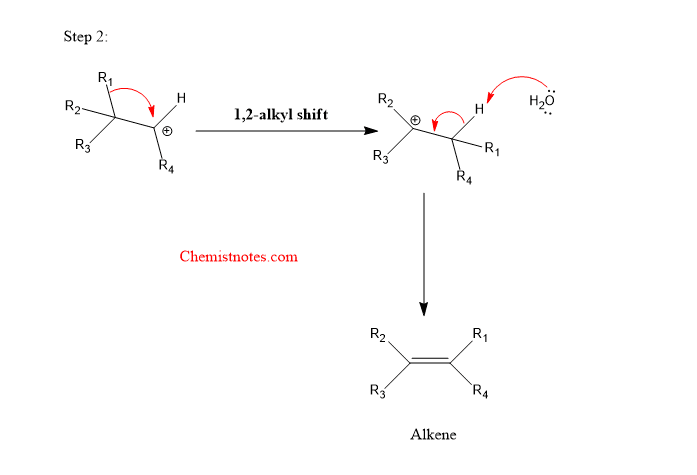
Step 2: 1,2-migration of alkyl group followed by loss of proton to give an alkene.
wagner meerwein rearrangement applications
The main application of Wanger meerwein rearrangements are given below:
- It is widely used in terpene chemistry
- It has been used to prepare phenanthrene.
- It can be used to prepare chiral auxillary in the organic chemistry.
wagner meerwein rearrangement video:
References:
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- J.J. Li, Name Reactions, 4th ed.,© Springer-Verlag Berlin Heidelberg, 2009
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010
If you want to learn about pinacol pinacolone rearrangement reaction, then click here. Please comment if you have any problems related to this topic. Thank you.







2 Responses
why is there a methyl shift in the second example since the carbocation formed after the protonation of the alcohol(secondary) is more stable than the one formed after the methyl shift (primary)
You are right Samson. Thanks for pointing out our mistake. Actually, we made mistake on that particular structure.