Table of Contents
TogglePinacol pinacolone rearrangement reaction, examples, mechanism, migratory aptitude, and application have been discussed here. This reaction was first of all reported by Fittig in 1860.
Pinacol pinacolone rearrangement reaction
Pinacol pinacolone rearrangement reaction is the acid-catalyzed rearrangement of vicinal diols to corresponding aldehyde or ketones. Vicinol diols are commonly called Pinacols, which undergoes 1,2-rearrangement in presence of acid to give carbonyl group. This reaction is generally carried out in presence of acid like H2SO4. Sometimes other acidic conditions are used for better yield of product such as HClO4, BF3 in acetic acid, ZnCl2 in acetic anhydride, H3PO4.
Let’s see a general example of this reaction:
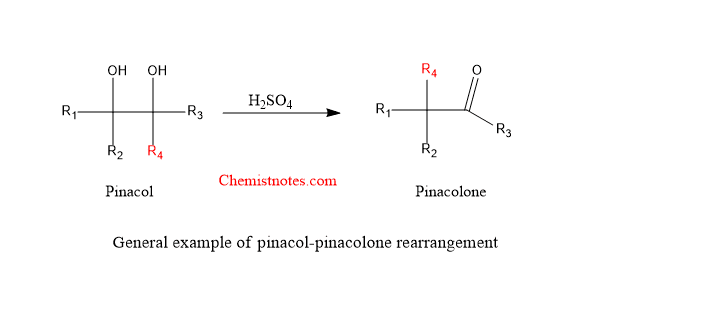
- Some molecules such as alpha-halohydrins,alpha-hydroxyepoxides and aziridines also undergo similar reaction. The arrangement of aziridine under similar condition is known as the aza-pinacol rearrangement.
Pinacol pinacolone rearrangement migratory aptitude
The regiochemistry of this reaction depends on the carbocation formed, the acid used, and the migratory aptitudes of the group on the vicinal diols. In the case of asymmetric pinacols, the decrease in the basicity of one hydroxy group by the neighboring group might facilitate the protonation of the other hydroxyl group.
The migratory aptitudes of substituents on diols are determined by their electron density and steric hindrance. It means the group having high electron density migrates than those groups with less electrons density.
The general order of migration of group is given below:
Tertiary alkyl>cyclohexyl>secondary alkyl>benzyl phenyl>primary alkyl> methyl>>H
Let’s see the order for substituted aryls:
P-MeO-Ar>P-Me-Ar>P-Cl-Ar>P-Br-Ar>P-MeOAr>P-O2N-Ar.
- If electron donating group such as methoxy, inctreases the migratory aptitude of the phenyl group at the para- position but the decrease the aptitude when present at the ortho-position.
Pinacol pinacolone rearrangement examples
Let’s see examples of pinacol pinacolone rearrangement reactions.
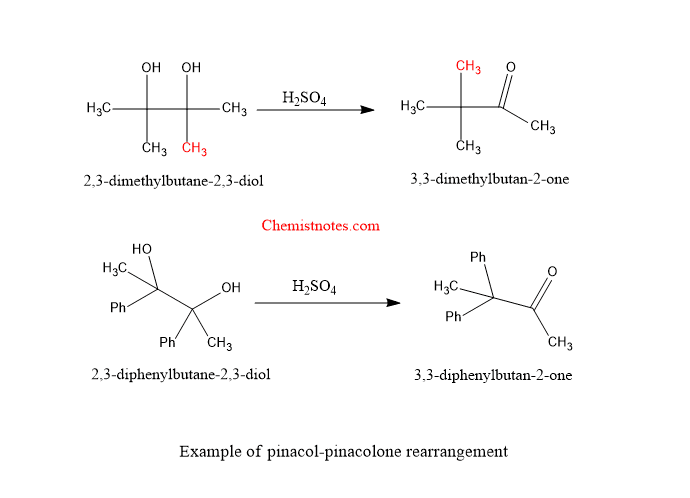
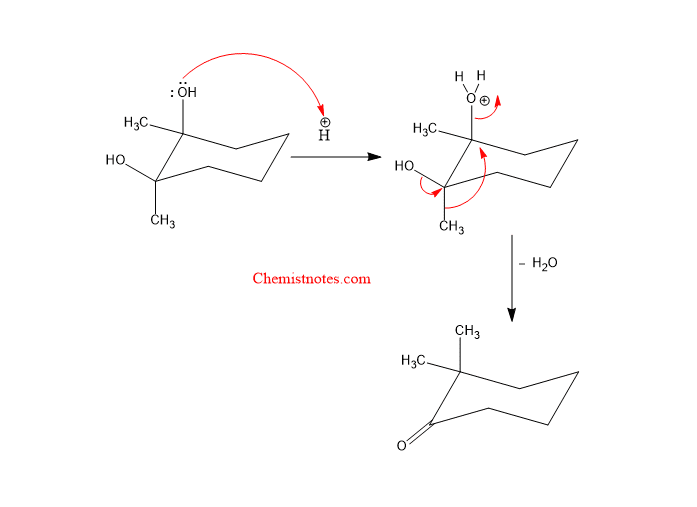
Reaction mechanism of pinacol pinacolone rearrangement
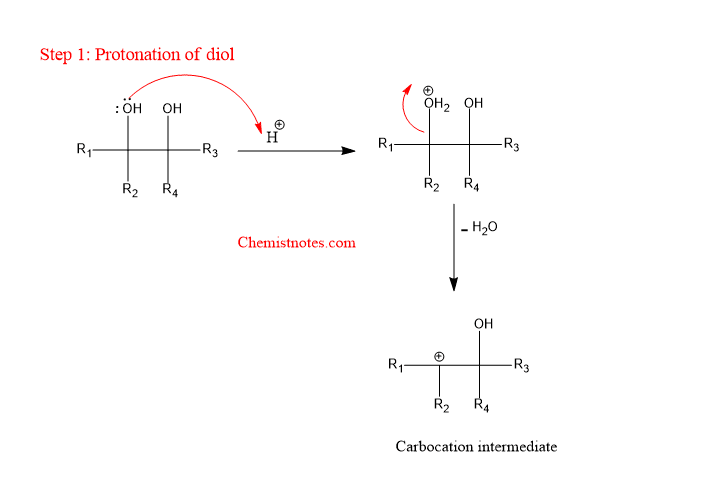
The pinacol pinacolone is a stepwise reaction that completes in the following two steps as shown below:
Step 1: Protonation of diol followed by the formation of a carbocation.
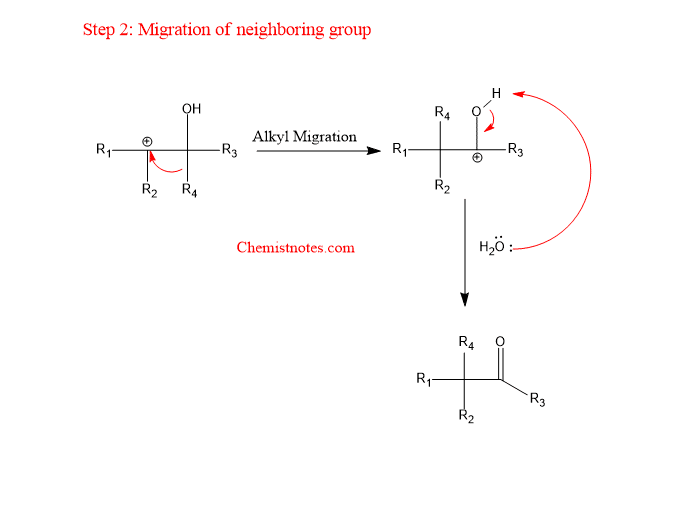
Step 2: Migration of a neighboring substituent to the carbocation carbon.
Pinacol pinacolone rearrangement in cyclic compounds
Let’s see some examples of pinacol pinacolone rearrangement in cyclic compounds.
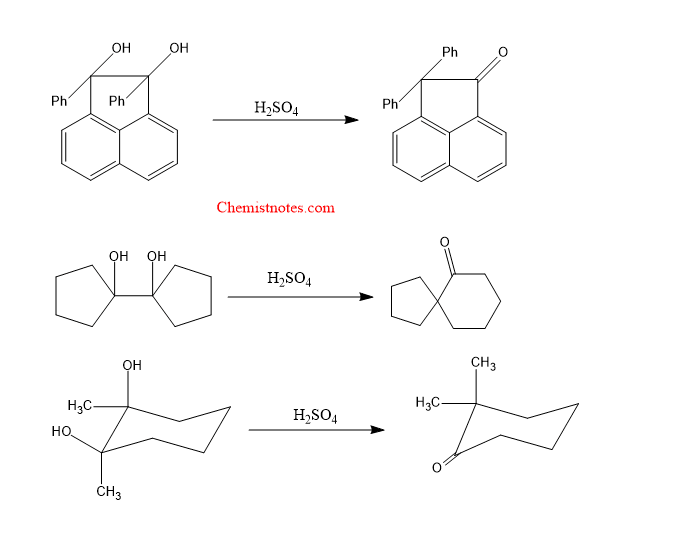
Let’s see the mechanism:
Application of pinacol pinacolone rearrangement
Pinacol pinacolone rearrangement has broad application in organic synthesis. It can be used to prepare pinacolone which is used in herbicides and many other drugs such as stiripentol, pinacidil, etc.
Pinacol pinacolone rearrangement Video:
References:
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010
- J.J. Li, Name Reactions, 4th ed.,© Springer-Verlag Berlin Heidelberg 2009
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987
If you want to learn about other rearrangement reactions such as Favorskii rearrangement, then click here.
Please comment if you have any problems related to this topic. Thank you.
FAQs/MCQs:
what is pinacol pinacolone rearrangement reaction?
Pinacol pinacolone rearrangement reaction is the acid-catalyzed rearrangement of vicinal diols to corresponding aldehyde or ketones.






