Table of Contents
TogglePerkin Condensation, examples, mechanisms, and applications in organic chemistry have been discussed here:
Perkin Condensation Reaction Definition
Perkin condensation reaction is the condensation reaction of an aromatic aldehyde and an aliphatic acid anhydride containing at least two α-hydrogen atoms in the presence of a sodium or potassium salt of the corresponding acid to form, α, β-unsaturated acid. Generally, such type of reaction is only applicable to aromatic aldehyde, and should be noted that only the anhydride’s α-hydrogen atoms are involved in the condensation.
Some of the examples of perkin reactions are:
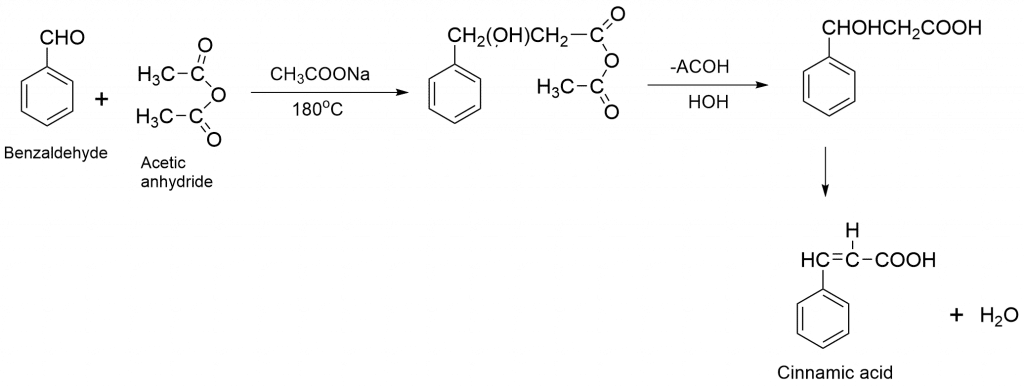
Perkin Condensation Mechanism
The aldol condensation of an aromatic aldehyde and an acid anhydride produces an α, β-unsaturated aromatic acid in the Perkin reaction. Here, the base abstracts the α-hydrogen atom and leads to the formation of the carbanion intermediate. The carbanion now attacks carbonyl carbon i.e. aldehyde by nucleophilic addition leading to the formation of α, β-unsaturated acid.
The perkin condensation mechanism takes place by multiple steps as:
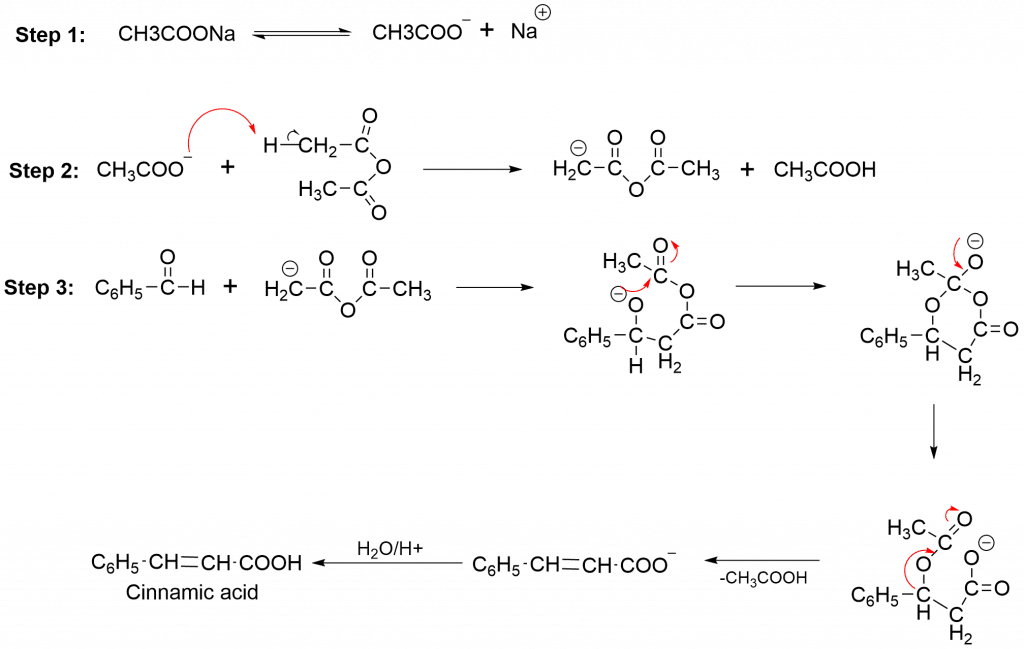
Applications of Perkin Condensation
1. Conversion of Furfural into Furylacrylic acid (65-70% yield)
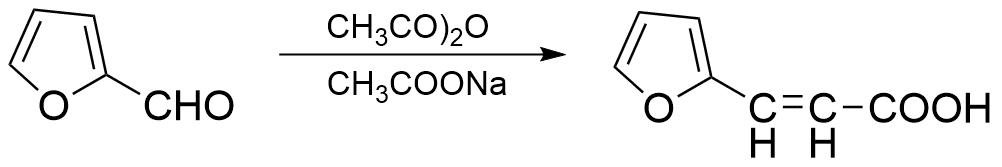
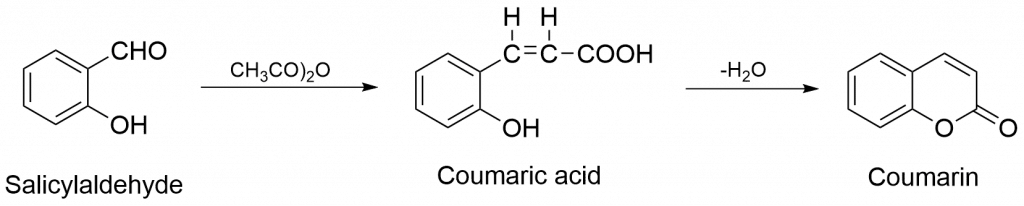
2. Formation of Coumaric acid from salicylaldehyde, which on dehydration yields Coumarin.
3. Synthesis of phytoestrogenic stilbene resveratrol
Perkin Condensation Video
FAQs/MCQs
What is perkin reaction?
Perkin reaction is a condensation reaction that involves the reaction of an aromatic aldehyde and an aliphatic acid anhydride containing at least two α-hydrogen atoms in the presence of a sodium or potassium salt of the corresponding acid to form, α, β-unsaturated acid.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.






