Regiochemistry in Birch reduction depends on the substituents present in the benzene or arene. The regiochemistry in birch reduction with a suitable explanation has been discussed in this post.
Regiochemistry in Birch Reduction
Birch reduction is the reduction of benzene or arene to 1,4-diene in presence of sodium metal dissolved in liquid ammonia.
If the benzene or arene contains substituents then the regioselectivity of the final product arises. It means, that when there are substituents around the aromatic rings, the questions of regiochemistry arise. In fact, it has been found that electron-withdrawing groups promote Ipso, para Birch reduction. Similarly, Electron donating group promotes ortho, meta Birch reduction.
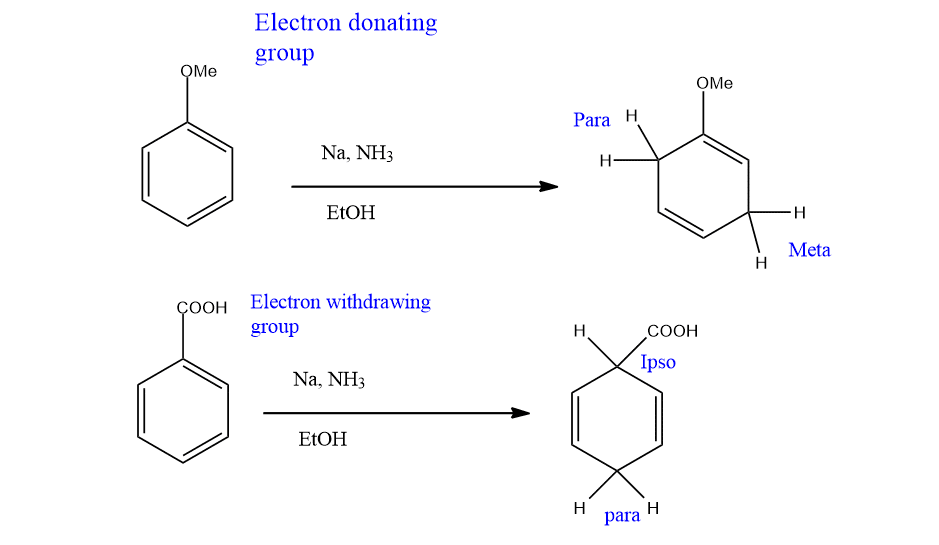
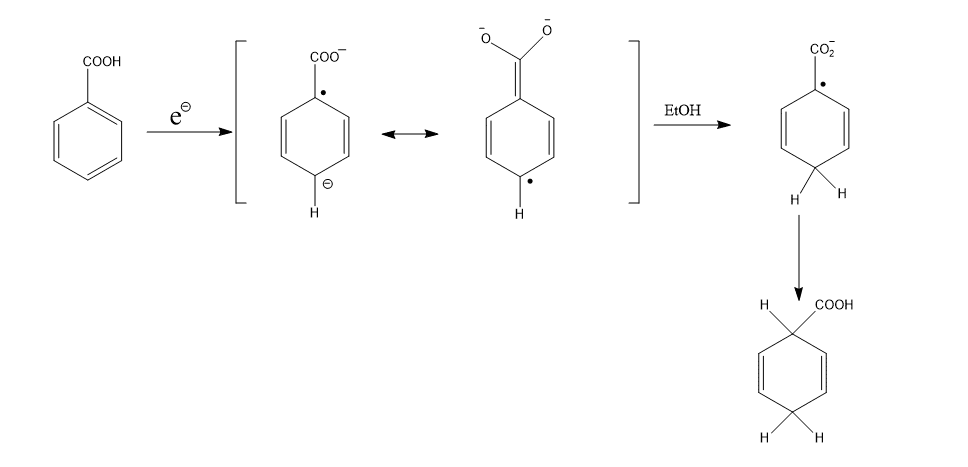
These can be explained on the basis of the distribution of electron density in the intermediate radical anions. Electron withdrawing groups stabilize electron density at the ipso and para positions.
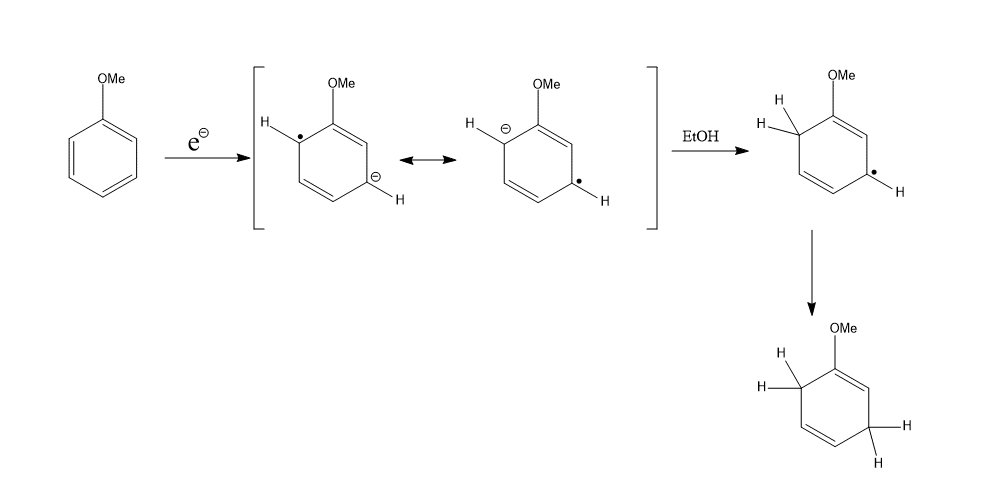
On the other hand, the electron-donating group stabilizes ortho and meta electron density.
References:
- Jonathan Clayden, Organic Chemistry, 2nd Edition, Oxford University Press, India






