Table of Contents
ToggleBirch reduction mechanism, examples, and applications in organic chemistry have been discussed here. It is one of the famous dissolved metal reductions. The first thing to notice is that when lithium or sodium dissolves in ammonia, they produce a bright blue solution. The blue color is due to the solvated electrons. Birch reductions use those blue solutions, with their solvated electrons, as reducing agents.
Birch Reduction definition
The reduction of aromatic hydrocarbons into cyclohexadiene in presence of Sodium or Lithium metal in liquid ammonia and solvent like ethanol is known as Birch reduction. This reaction was named after Arthur Birch, who was an Australian chemist. Alcohol likes ethanol acts as a proton donor here.
Some birch reduction examples are shown below:
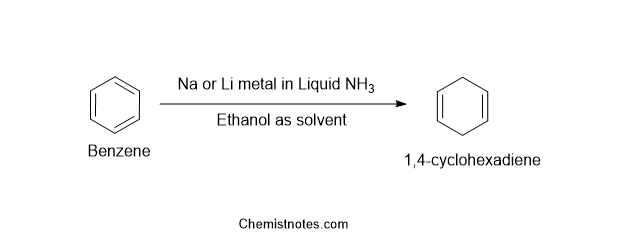
Birch Reduction mechanism
Now, let’s explore the mechanism of birch reduction. The steps involved in the reactions are given in stepwise manners.
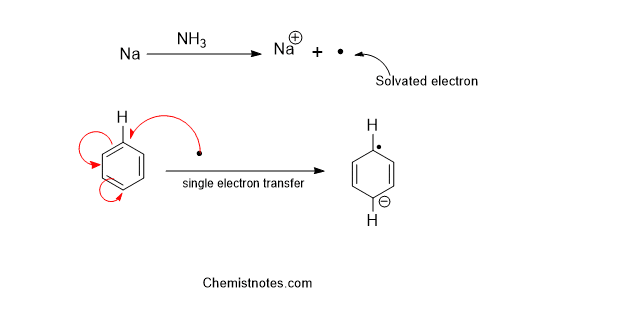
Step 1: Single-electron transfer from sodium metal to the aromatic ring to form a radical anion.
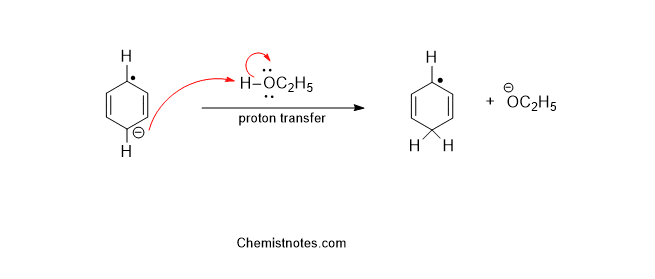
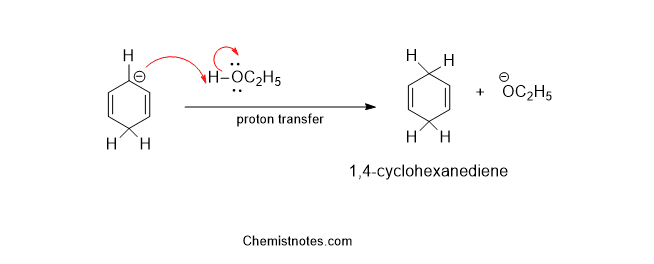
Step 2: Proton transfer takes place from the solvent to the radical anion.
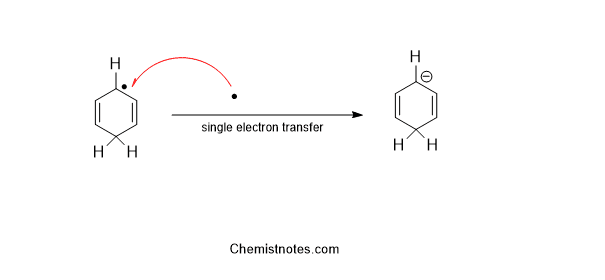
Step 3: In this step, a single electron is transferred from sodium metal to radical.
Step 4: In the last step, a proton is transferred from the solvent to the anion formed in the above step.
Effect of Substituents in Birch Reduction
Let’s explore some ideas about the effect of substituents in the Birch reduction.
What happens when the aromatic ring has an electron-donating or electron-withdrawing group? Aromatic rings have such substituents when undergoing birch reduction, the products formed are different in each case.
In birch reduction, solvated electron acts as a nucleophile, and hence the presence of electron-withdrawing group on the aromatic rings increases the reaction rate. But the presence of an electron-donating group on the rings decreases the rate of reaction because the electron-donating group decreases positive character in the ring.
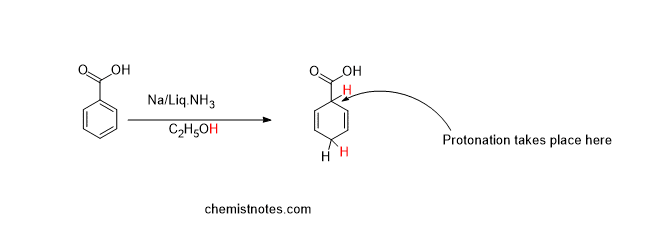
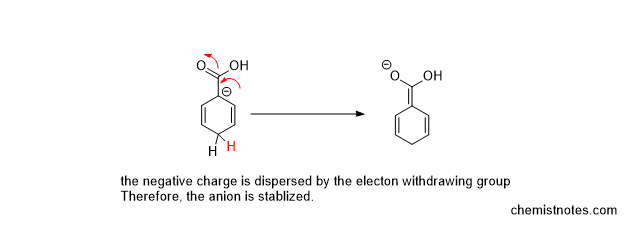
Effects of electron withdrawing group in birch reduction
If the aromatic rings containing the electron-withdrawing group (such as the COOH group) undergo birch reduction, protonation takes place at the carbon-containing electron-withdrawing group, which is illustrated below:
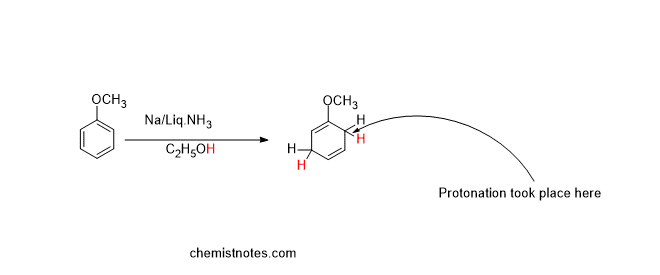
Effect of electron-donating group in birch reduction
If the aromatic rings containing the electron-donating group (such as the OCH3 group) undergo birch reduction, protonation takes place at the ortho position to the electron-donating group, which is shown below:
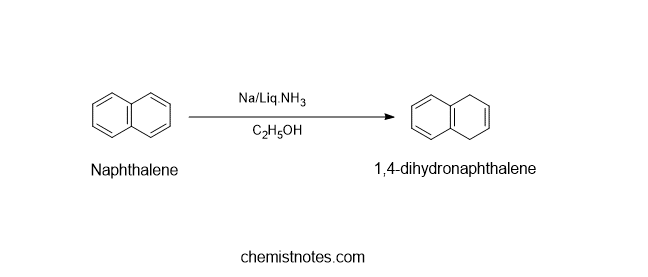
Birch reduction of naphthalene
When naphthalene is reduced in presence of sodium in liquid ammonia and alcohol, naphthalene is converted into 1,4-dihydronaphthalene.
Application of birch reduction
Some of the important birch reduction application is given below:
A. Birch reduction is used mainly for the reduction of aromatic compounds since it selectively reduces double bonds present in the compounds.
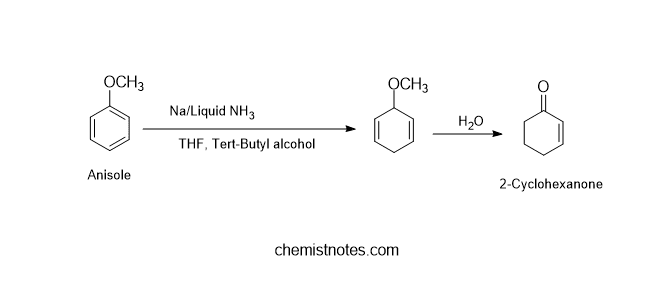
B. Birch reduction of anisole
Anisole can be reduced to 1-methoxy-1,4-cyclohexadiene by using birch reduction, which on further hydrolysis gives 2-cyclohexanone.
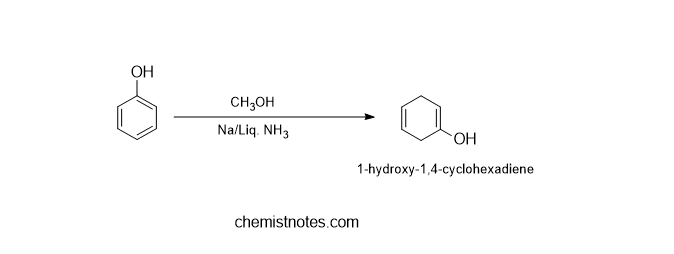
C. Reduction of phenol
Phenol can be reduced to 1-hydroxy-1,4-cyclohexadiene using Birch reduction
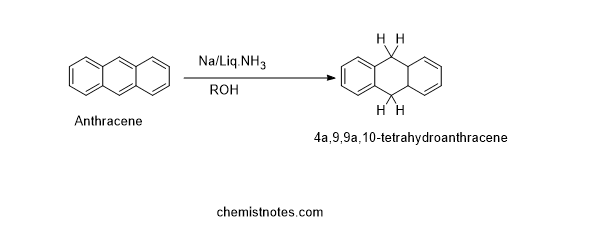
Birch reduction of Anthracene
The birch reduction of anthracene gives tetrahydro anthracene, which is given below:
FAQs/MCQs:
What is Birch reduction?
Birch reduction is a type of reaction in which the double bond of aromatic compounds is reduced selectively in presence of sodium or lithium in liquid ammonia and alcohol.






