Table of Contents
ToggleReformatsky reaction, examples, mechanism, and its application have been discussed here. This reaction was first of all, reported by Reformatsky in 1887.
Reformatsky reaction
Reformatsky reaction is the reaction between alpha-bromoester and an aldehyde or ketone in presence of Zinc metal to give β-hydroxy ester. This is a nucleophilic addition reaction between α-haloester and a carbonyl compound. This reaction is also known as Reformatsky condensation or Reformatsky addition.
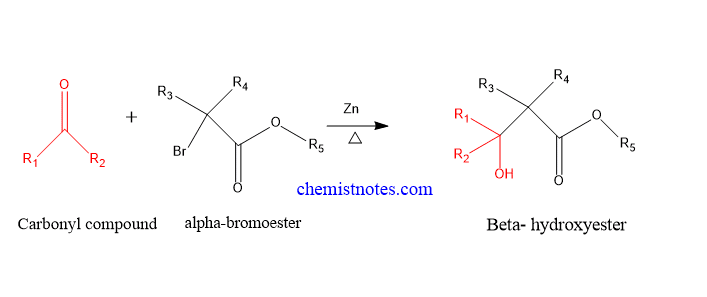
First of all, α-haloester reacts with zinc metal to form an organozinc reagent, which undergoes nucleophilic addition reaction to aldehyde or ketone. The reaction between the Zinc metal and α-haloester gives an inserted complex known as Reformatsky reagent.
Due to the delayed initiation of zinc metal in typical Reformatsky reactions, various types of activated zinc, such as finely divided acid-washed, granular zinc, and so on, have been used.
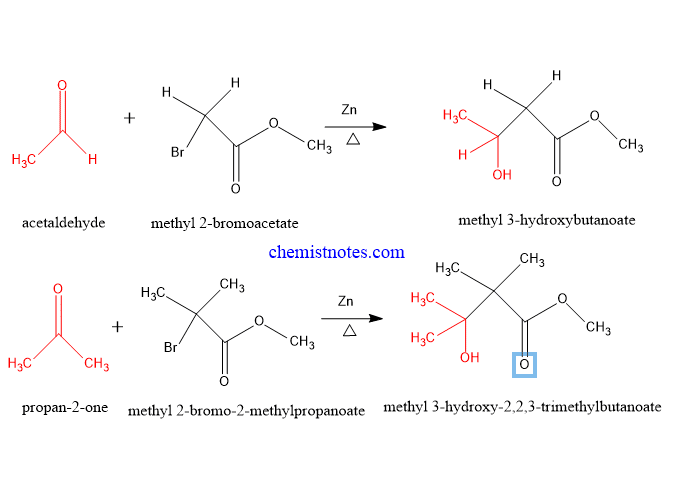
Example of reformatsky reaction
Some examples of this reaction are given below:
reformatsky reaction mechanism
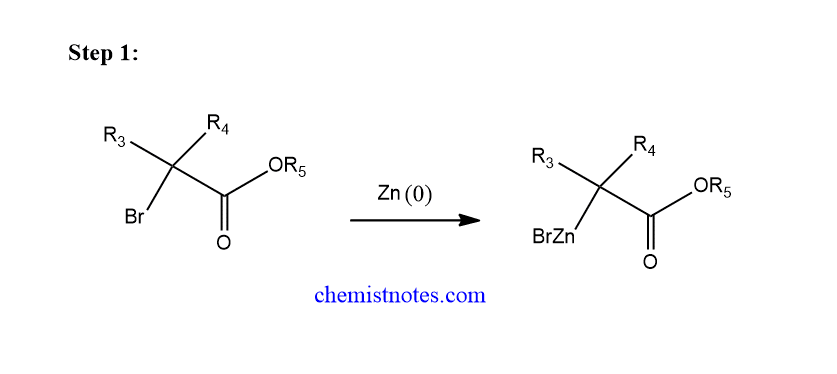
The mechanism of the Reformatsky reaction completes in the following 3 steps.
Step 1: Oxidative addition of Zinc metal to α-haloester to form reformatsky reagent. This is actually an electron transfer reaction.
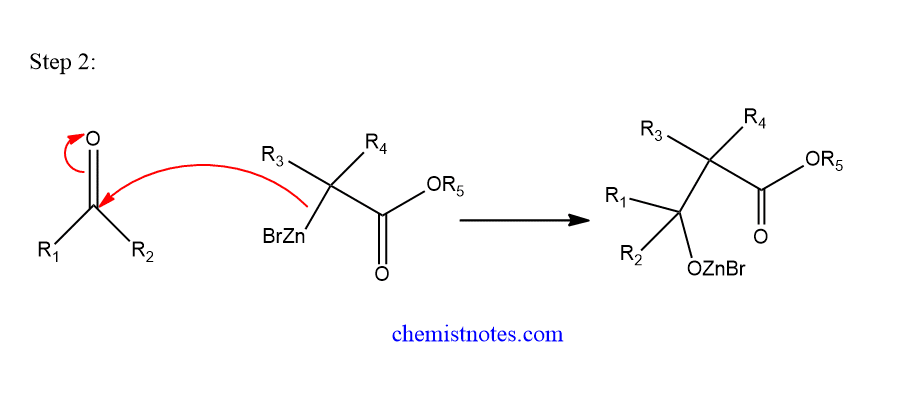
Step 2: Nucleophilic addition of reformatsky reagent to the carbonyl compound and form adduct.
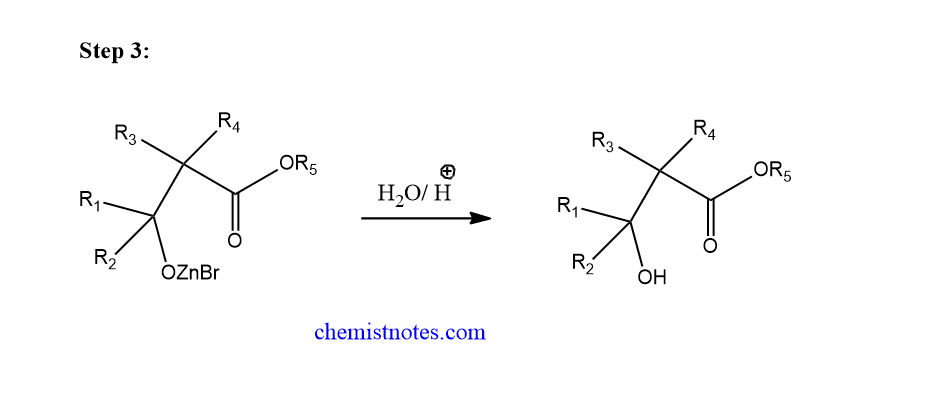
Step 3: Hydrolysis of addition adduct
Uses of reformatsky reaction
The main application of the reformatsky reaction is to prepare β-hydroxy ester from aldehyde or ketones.
References:
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010
- J.J. Li, Name Reactions, 4th ed.,© Springer-Verlag Berlin Heidelberg 2009
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987
Please comment down in the comment box if you find any mistakes. If you want to learn more about name reactions such as Stork enamine reaction, then click here.
FAQs/MCQs:
Will the reformatsky reaction work with a ketone?
Yes, reformatsky reaction work with carbonyl compounds, both aldehyde, and ketones.
Why magnesium is not used in reformatsky reactions?
When magnesium is used in a reformatsky reaction, it will form a Grignard reagent by reacting with ester, which will react with other ester molecules and the whole process is interrupted. Hence, magnesium is not used in reformatsky reactions.






