Table of Contents
ToggleMitsunobu reaction mechanism, examples, intramolecular Mitsunobu reaction, and application have been discussed here. This reaction was first of all reported by Mitsunobu in 1967.
Mitsunobu reaction
Mitsunobu reaction is the reaction between the primary or secondary alcohol with a compound with an active proton in presence of triphenylphosphine and Diethyl azodicarboxylate(DEAD) or Diisopropyl azodicarboxylate (DIAD) to form esters, ethers, amines, or thioether. The final result is determined by the compound/nucleophile utilized.
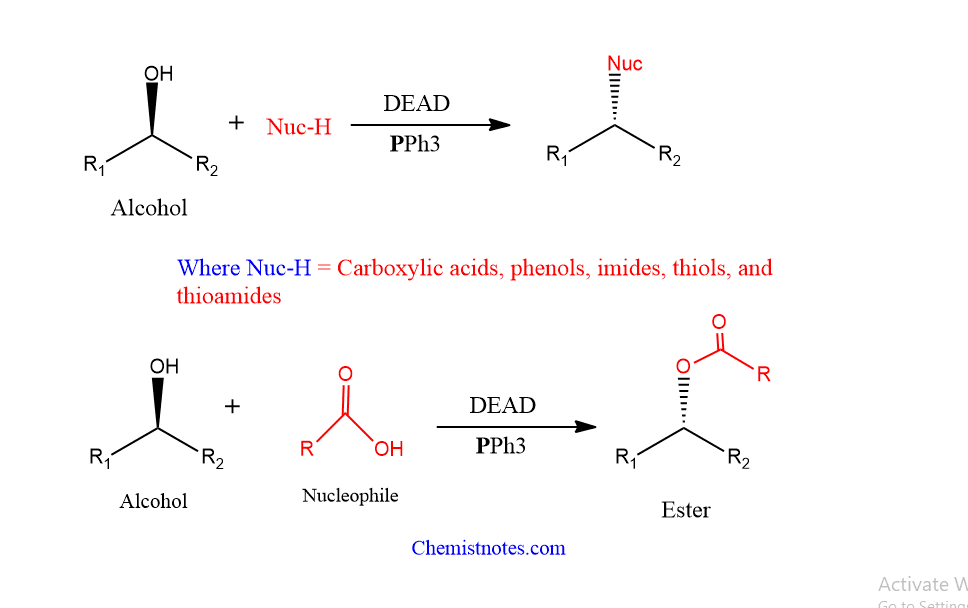
- This reaction is also known as the Mitsunobu coupling reaction
- The product’s sterochemistry is reversed.
- Such reactions take place in a neutral medium at room temperature.
- Mitsunobu esterifcation is the name given to a reaction in which the ultimate product is an ester.
- Primary and secondary alcohols are good starting materials for the Mitsunobu reaction. Tertiary alcohols are less suited because they are poor SN2-mechanism substrates.
- Carboxylic acids, phenols, imides, thiols, and thioamides are among the nucleophiles that can be used.
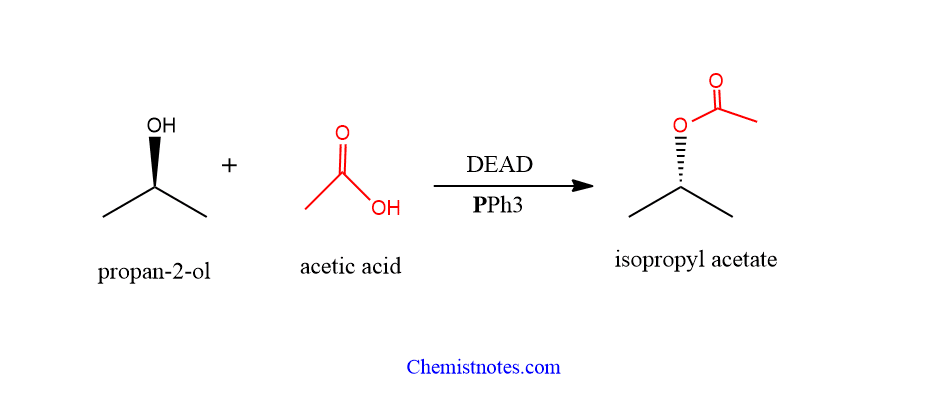
mitsunobu reaction examples
Let’s see some examples of this reaction.
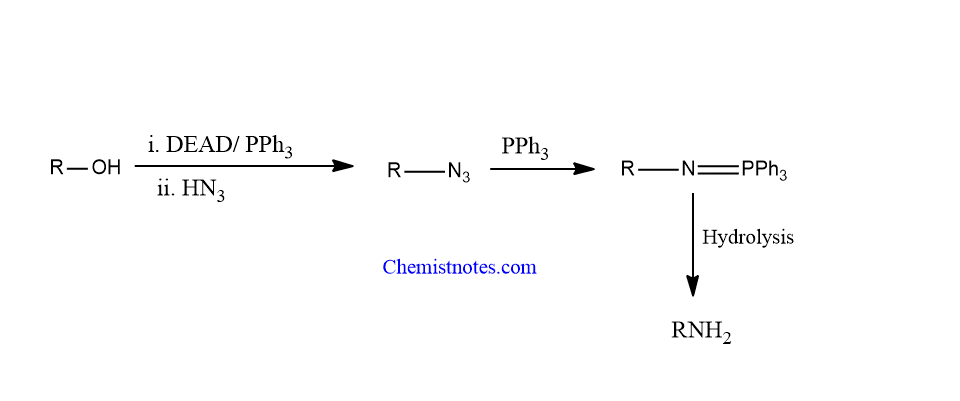
Similarly, In a reaction using hydrazoic acid (HN3), excess triphenylphosphine, and diethyl azodicarboxylate, alcohol can be converted to an amine.
mitsunobu reaction mechanism
The mechanism of the mitsunobu reaction completes in the following steps.
Step 1: Nucleophilic addition of triphenylphosphine on N=N double bond of DEAD to form betaine, also known as Morrison Brunn-Huisgen intermediate.
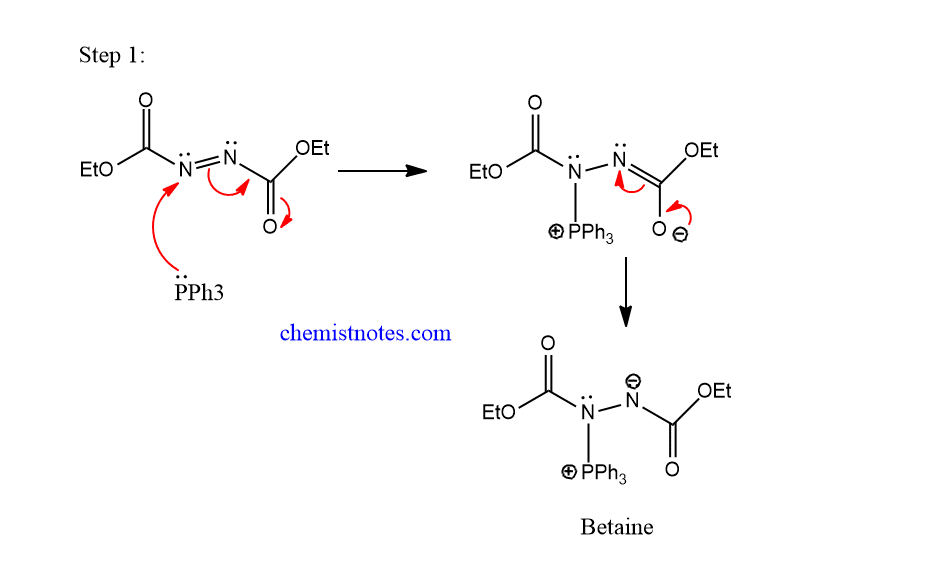
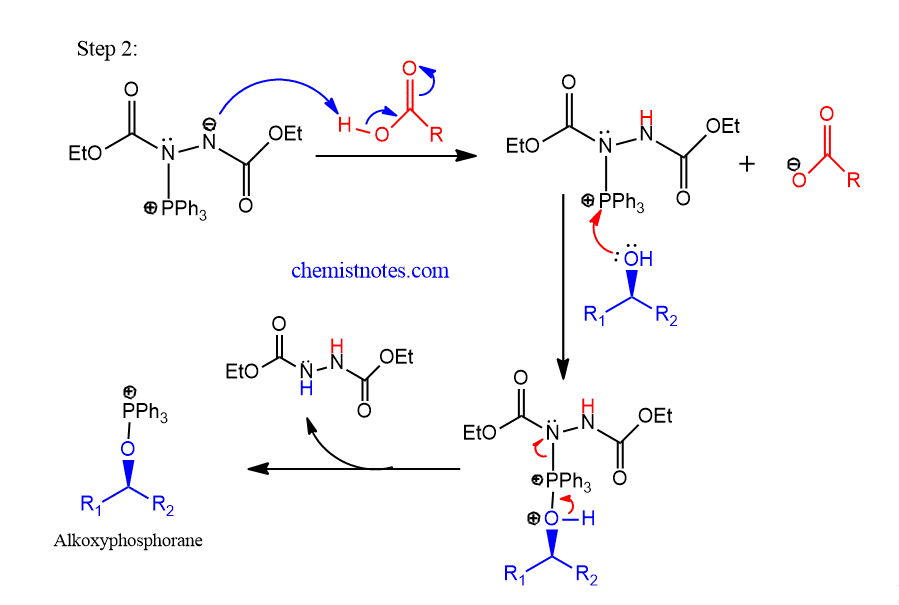
Step 2: Betaine intermediate abstracts a hydrogen atom from the nucleophile having active proton to form alkoxyphosphorane.
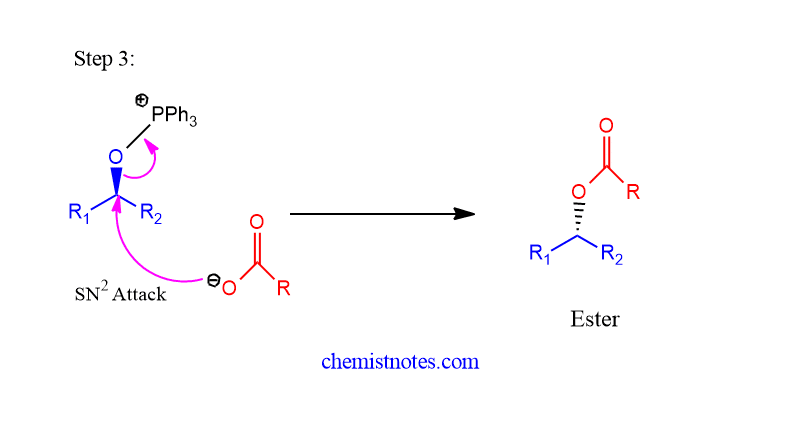
Step 3: The alkoxyphosphorane undergoes an SN2 reaction with a nucleophile to give product.
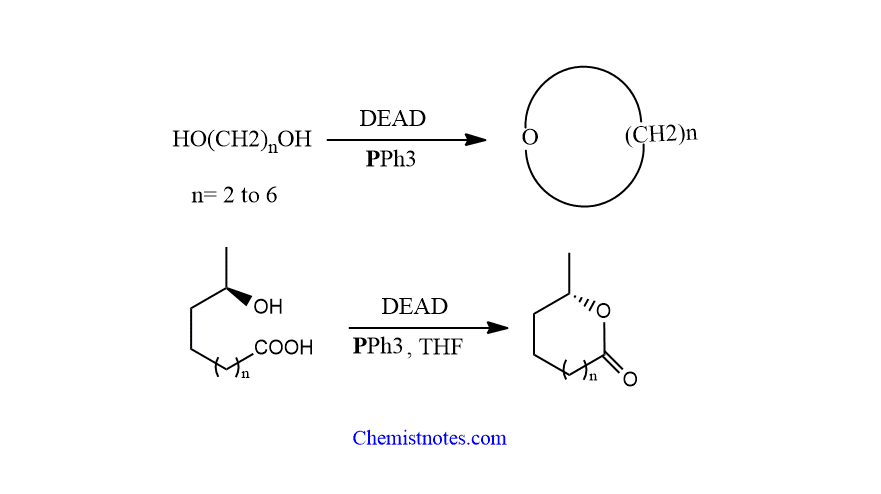
intramolecular mitsunobu reaction
Intramolecular mitsunobu reaction is generally observed in compounds such as diols or hydroxy acids compounds. An intramolecular mitsunobu reaction can be used to make cyclic ethers, which is particularly useful for the synthesis of three- to seven-membered rings. Let’s see some examples.
Application of mitsunobu reaction
The major application of Mitsunobu reaction is the preparation of ester with inversion of configuration from a secondary alcohol. Alcohols can be transformed into various types of molecules such as amines, ethers, and azides by using the right nucleophiles.
Mitsunobu reaction video
References:
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010
- J.J. Li, Name Reactions, 4th ed.,© Springer-Verlag Berlin Heidelberg 2009
- Bradford P. Mundy, Michael G. Ellerd, Frank G. Favaloro, Jr., Name Reaction and Reagents in Organic Synthesis, John Wiley & Sons, 2005
- Laue T.,Plagens A.,Named Organic Reactions, Second edition, John Wiley & Sons, Ltd, 2005
If you have any problem related to this topic, you can comment without any hesitation. Thank you, Happy Learning.






