Table of Contents
ToggleWittig reaction mechanism, examples, and applications in organic chemistry have been discussed here:
Wittig Reaction Definition
Wittig reaction involves the reaction of aldehydes or ketones (aliphatic or aromatic) with wittig reagents to produce olefin. Triphenylphosphinealkylidenes or alkylidenephosphoranes are widely used as wittig reagents. Because alkylidenephosphoranes react as quickly with molecular oxygen as they do with oxygen-containing molecules, the Wittig reaction is carried out in an oxygen-free nitrogen environment.
This is a typical method for synthesizing alkenes in organic chemistry. The geometry of the resultant alkene is determined by the ylide’s reactivity. When R is an electron-withdrawing group, the ylide becomes more stable and less reactive, but the reverse happens when R is an alkyl group (electron releasing). Non-stabilized ylides produce (Z)-alkenes, whereas stabilized ylides produce (E)-alkenes.
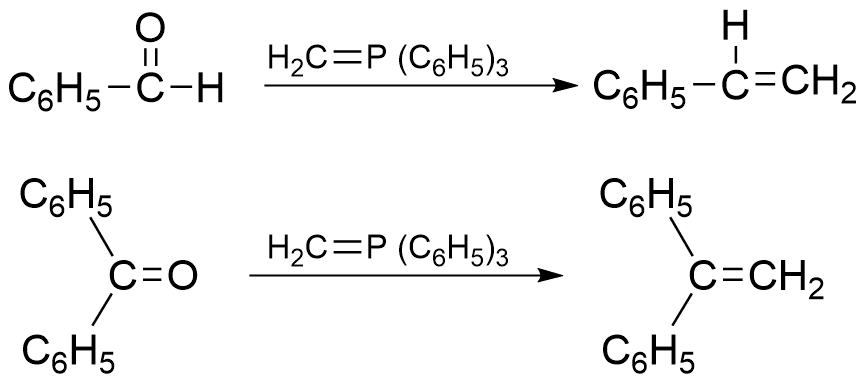
Wittig reaction examples
Some of the examples of Wittig reactions are:
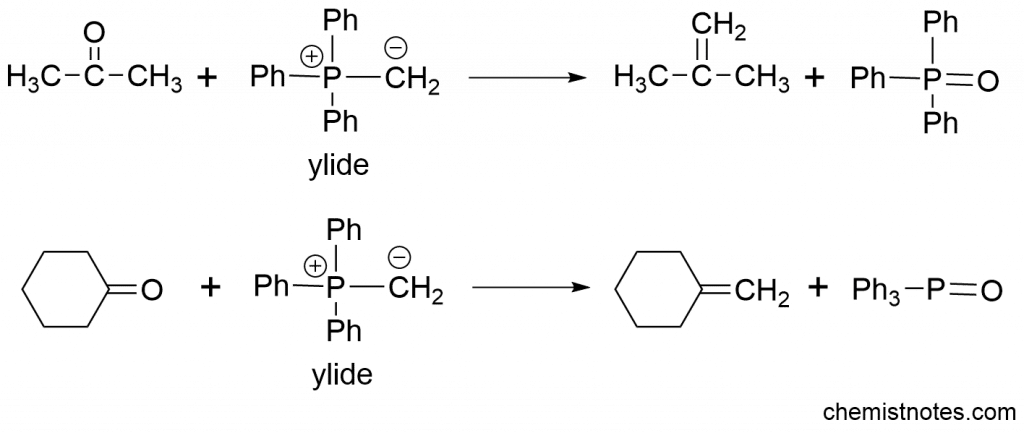
Wittig reaction mechanism
The Wittig reaction mechanism can be carried out into 3 steps:
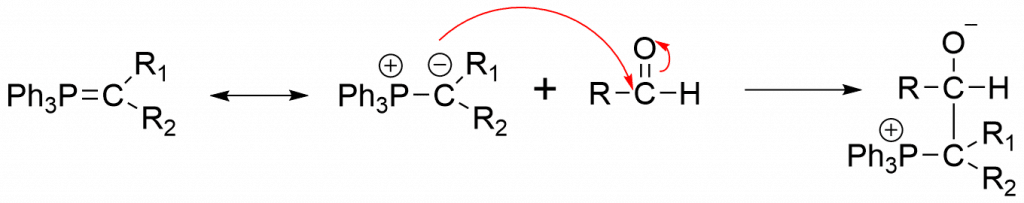
Step 1: The anionic carbon of the ylide undergoes nucleophilic attack on the electron-deficient carbonyl carbon, yielding betaine as an intermediate.
Step 2: P-O bond is formed through a cyclic transition state in order to yield a cyclic compound.
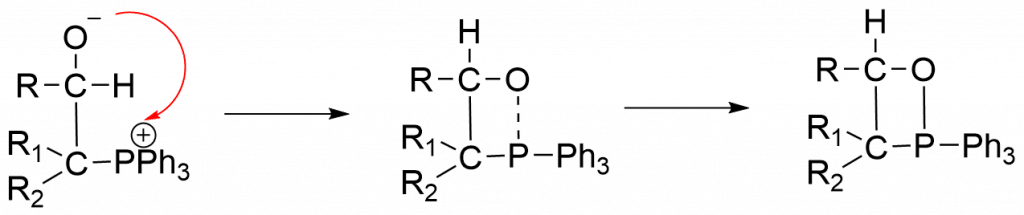
Step 3: Breaking of the C-O and C-P sigma bonds and formation of the C-C sigma bonds via a four-centered cyclic transition state.
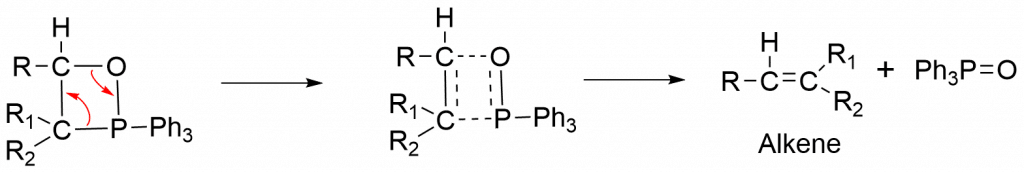
Applications of Wittig reaction
Wittig reaction is mostly used for the preparation of alkenes where the position of the double bond is fixed.
Advantages of Wittig reaction
- If the Ylide’s nature is known, the geometry of the double bond is easily determined.
- Large number of alkenes can be synthesized via this method.
Limitations of Wittig reaction
Some of the major limitations of Wittig reactions are:
- Aldehydes can be easily oxidized, decomposed, or even polymerized.
- It is possible to obtain both the E and Z double bond isomers.
Wittig reaction Video
FAQs/MCQs
Importance of wittig reaction
Wittig reaction is widely used for synthesizing alkenes in organic chemistry.
What is wittig reaction?
The reaction in which aldehyde or ketone reacts with triphenylphosphine alkylidenes to produce olefin is called the wittig reaction.
Wittig reagent
Triphenyl phosphonium ylide is used as wittig reagent
Wittig reaction problems
The reaction becomes slow when sterically hindered ketone is used, and hence leads to the low yield. It is considered as one of the wittig reaction problems.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.






