Table of Contents
ToggleRetro diels alder reaction, mechanism, application, intramolecular diels alder reaction, hetero diels alder, and inverse electron demand diels alder reaction have been discussed here.
Retro diels alder reaction
Retro diels alder reaction is the opposite of the Diels-Alder reaction, in which an organic molecule containing a double bond in a six-membered ring undergoes cycloreversion to produce a diene and a dienophile. As a result, it’s the inverse of diels alder cycloaddition. It is generally known as Retro diels alder fragmentation or inverse diels alder reaction.
retro diels alder reaction examples
The example of retro diels alder reaction is shown below:
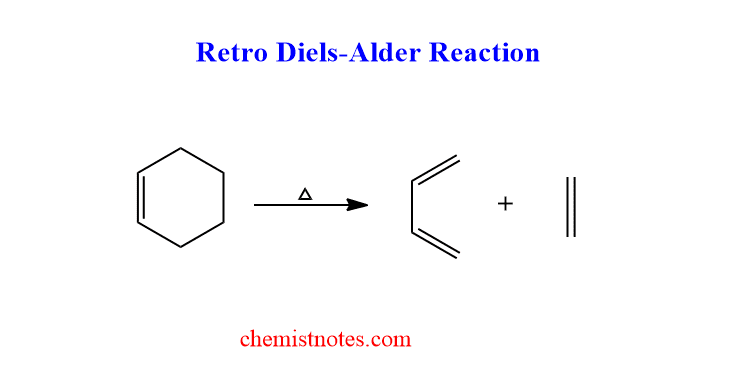
retro diels alder reaction mechanism
The mechanism of this reaction is a concerted mechanism that completes as shown below:
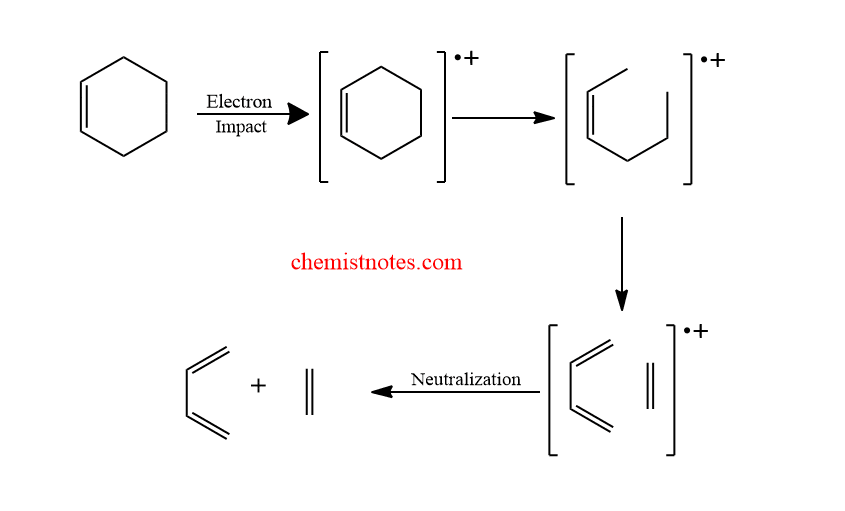
The electron-withdrawing group on the resultant dienophiles and the electron-donating group on the diene help to accelerate this process.
Application of retro diels alder reaction
Some application of retro diels alder reaction is listed below:
- It has a wide range of uses in chemical synthesis, including the generation of quinones, benzoquinones, and heterocycles.
- Double bonds can be protected using this reaction.
Intramolecular diels alder reaction
When the same molecule contains both dienes and dienophiles in the structure then such molecules undergo intramolecular diels-alder reaction. Intramolecular diels alder reaction gives a bicyclic product. Let’s see an example of an intermolecular diels alder reaction.
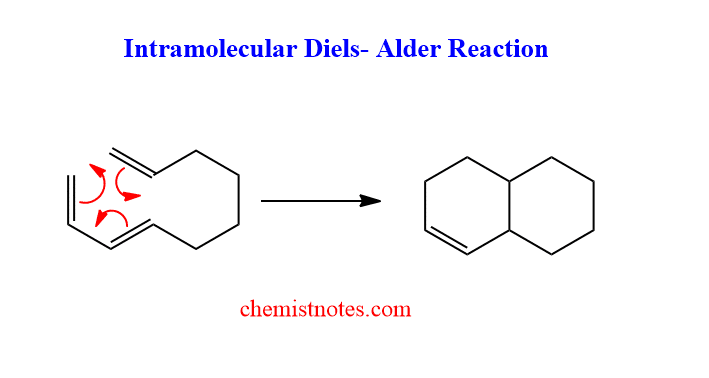
Inverse electron demand diels alder reaction
The dienophile has an electron-donating group (EDG) and the diene has an electron-withdrawing group (EDG) in this reaction (EWG). Therefore, The electron environment is the absolute opposite of a normal diels-alder reaction. Diene is an electron-poor species, whereas dienophile is an electron-rich species, and the two react to produce a six-membered adduct. Such reaction is termed as inverse electron demand diels alder reaction.
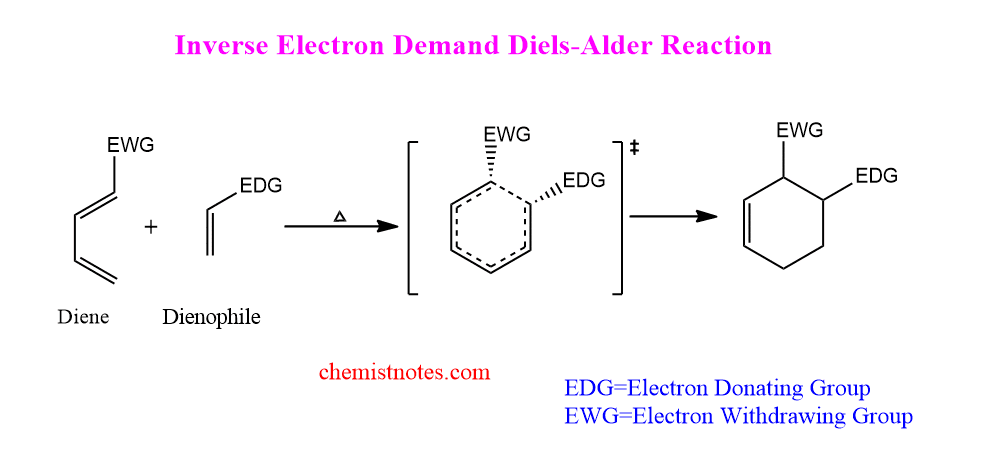
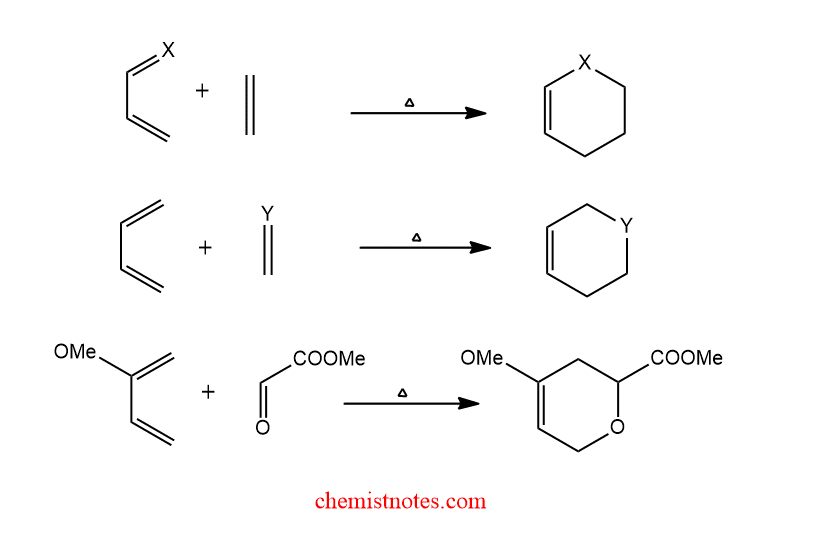
Hetero diels alder reaction
The process is called hetero diels alder if the atoms involved in the ring formation are not carbon atoms only. One or more heteroatoms serve as the center for the generation of six-membered heterocycles. The aza-diels alder reaction and the oxo-diels alder reaction are two examples of hetero diels alder reactions.
Let’s see some general examples of hetero diels alder reaction.
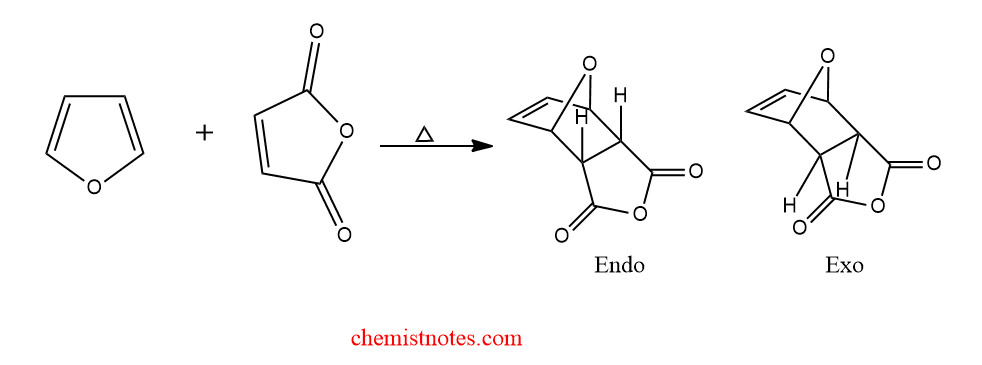
diels alder reaction between furan and maleic anhydride
When furan reacts with maleic anhydride to give endo product initially, which later converted to exo-product.
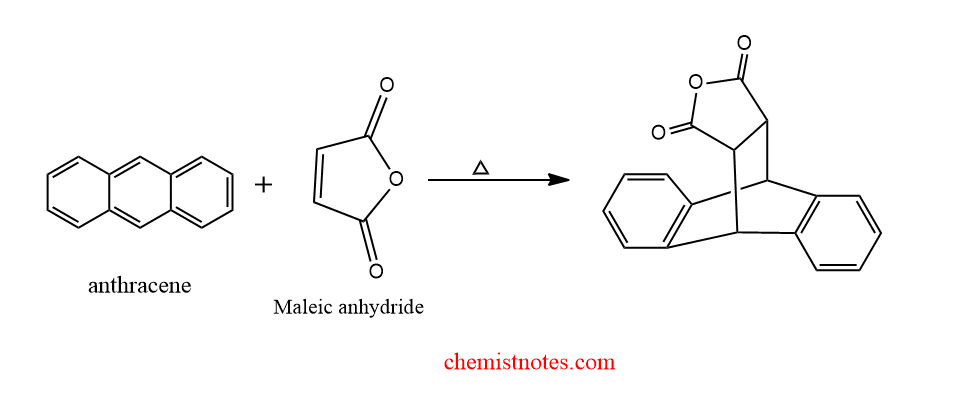
diels alder reaction of anthracene with maleic anhydride
The diels alder reaction of anthracene with maleic anhydride is shown below:
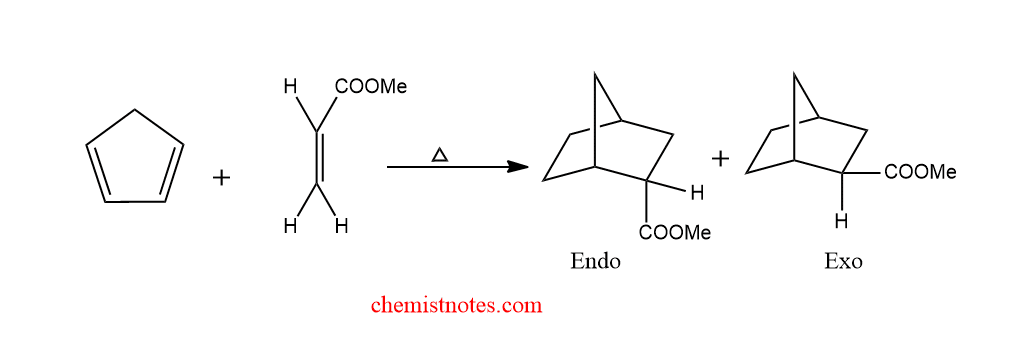
diels alder reaction cyclopentadiene
Let’s see an example of the diels alder reaction of cyclopentadiene.
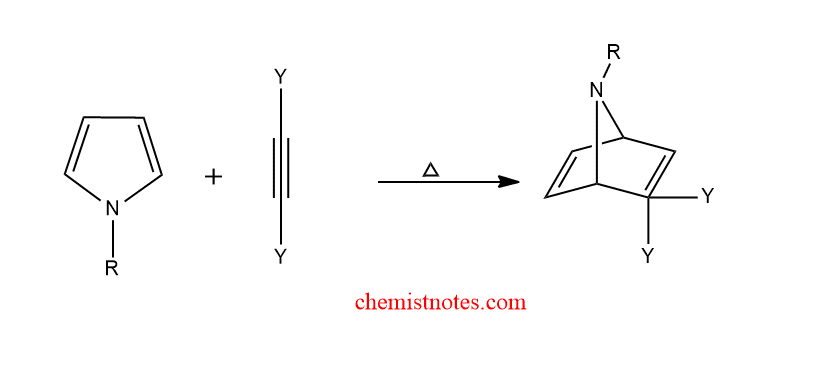
diels alder reaction of pyrrole
Pyrrole undergoes diels alder reaction with alkene and alkyne to give a bicyclic product.
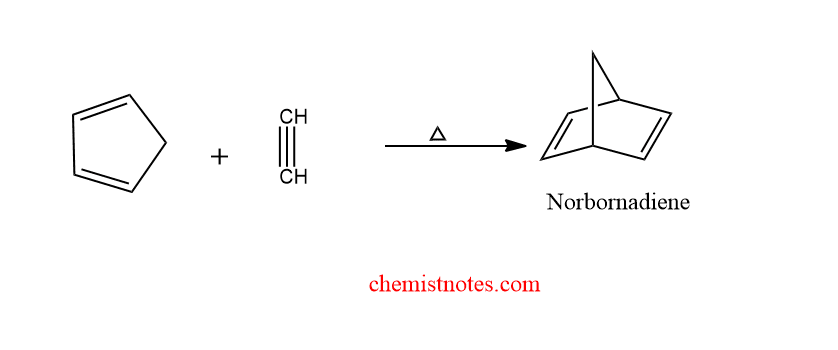
diels alder reaction with alkyne
When alkyne undergoes diels alder reaction with cyclopentadiene to give norbornadiene. Here, alkyne acts as a dienophile, and cyclopentadiene acts as diene.
References:
- Laue T.,Plagens A.,Named Organic Reactions, Second edition, John Wiley & Sons, Ltd, 2005
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010
- J.J. Li, Name Reactions, 4th ed.,© Springer-Verlag Berlin Heidelberg 2009






