Table of Contents
TogglePaterno Buchi reaction mechanism was first of all reported by Paterno and Chieff in 1909 and later extended by Buchi in 1954. The [2+2] photocycloaddition reaction of a carbonyl compound with an alkene is called the Paterno Buchi reaction. This reaction is also known as Paterno-Buchi cycloaddition or Paterno-Buchi Photocycloaddition or Paterno-Buchi coupling.
Paterno buchi reaction
The photo-induced [2+2] cycloaddition of carbonyl compounds with alkene or olefin or other unsaturated substrates to produce four-membered cyclic ether, oxetane is known as Paterno Buchi reaction.
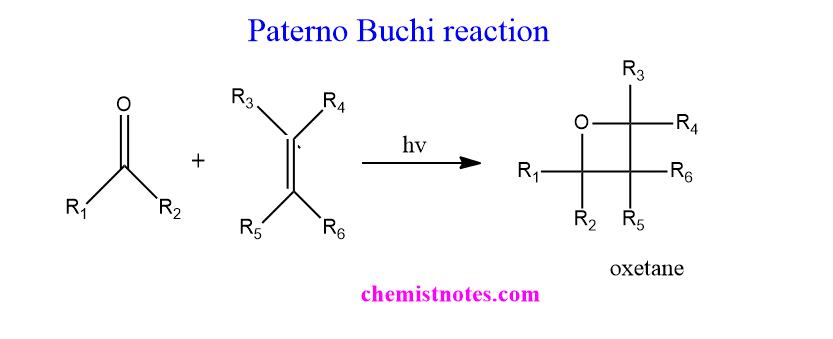
All kinds of ketones such as dialkyl or diaryl or aryl alkyl ketones as well as aldehydes undergo this types of reaction and some other types of photoreactions such as Norrish types I and Norrish type II reactions. When carbonyl compound is irradiated with the light near UV, excited carbonyl species is formed by n to π* transition.
It is generally found that which types of excited species(triplet or singlet) are formed depends on substrates. For example, aromatic carbonyl compounds generally are excited to the triplet excited state, and aliphatic carbonyls compounds are excited to the singlet state. Moreover, This reaction occurs with high regioselectivity and stereoselectivity.
Paterno buchi reaction examples
One example of the Paterno Buchi reaction is shown below.
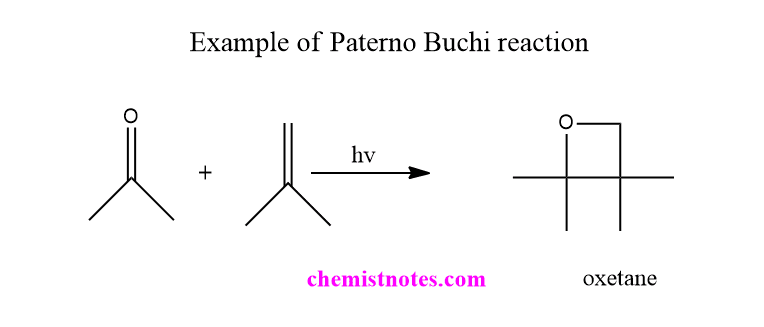
Paterno buchi reaction mechanism
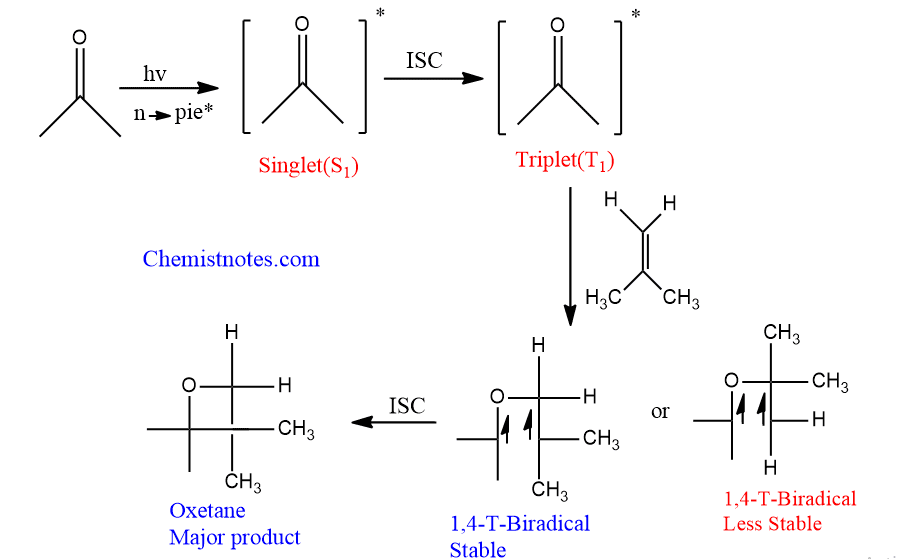
The carbonyl compound is irradiated to either a singlet or triplet excited state. The formed singlet state undergoes intersystem crossing to form a triplet excited state.
If the alkene is electron-rich, the triplet state carbonyl compound attack that alkene in a nonconcerted manner to form a biradical intermediate. Two possible biradicals are formed in this process, among them stable one gives oxetane as a major product, and less stable biradical forms minor product.
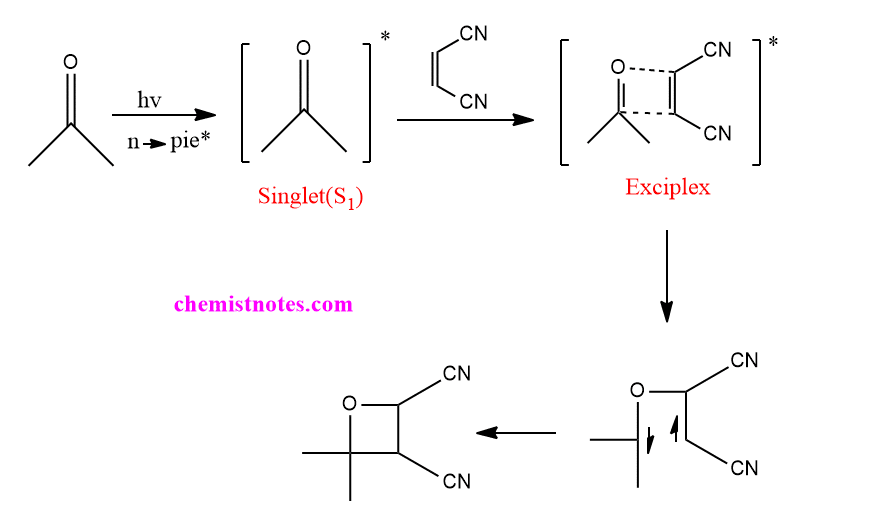
But if olefin is electron-deficient species such as dicyanoethane, the singlet excited state( n→π*) carbonyl compound reacts with ground state alkene to form exciplex as intermediate, which gives oxetane as a product. The reaction is stereospecific and the stereochemistry of the alkene is retained in the product oxetane.
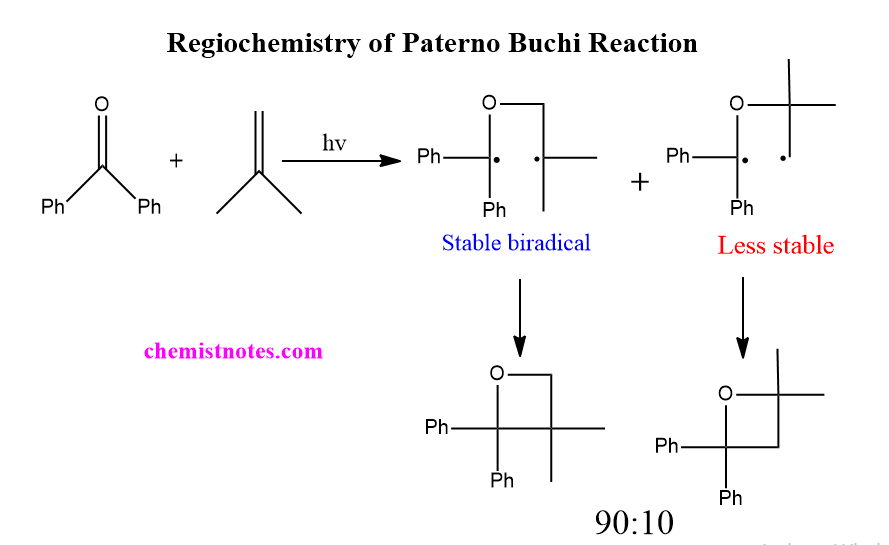
Regiochemistry of paterno buchi reaction
Paterno-Buchi reaction is a regioselective reaction. This can be explained in terms of the initial attack of the oxygen atom of the excited state carbonyl compound on the alkene. When excited state carbonyl compound attacks ground state alkene, biradical intermediate is formed. Since two types of the orientation of addition are possible, two types of biradicals are possible. The more energetically stable among two biradicals are formed. i.e the more stable biradical is generated. The stable biradical then gives oxetane as a product. Thus, the Paterno buchi reaction is a regioselective reaction.
For example, The photoaddition reaction of benzophenone to isobutene gives two regiomers in a 90:10 ratio. The most biradical gives major product, which is illustrated below:
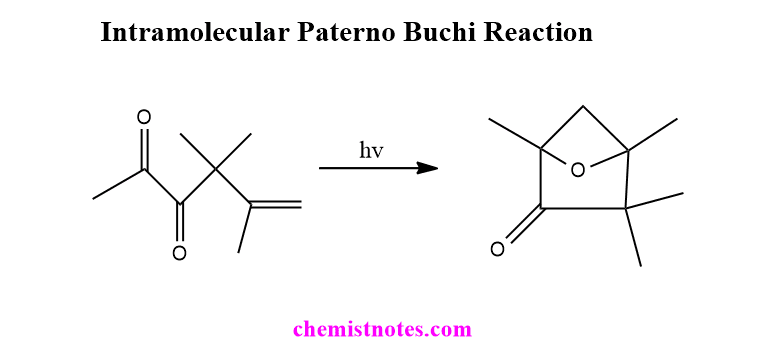
Intramolecular paterno buchi reaction
Some compounds having carbonyl group and carbon-carbon double in appropriate position can undergo intramolecular Paterno Buchi reaction in presence of light. Let’s have an example.
Application of paterno buchi reaction
Paterno Buchi reaction has wide application in organic synthesis. It has been used in the synthesis of natural products in recent years. Not only in organic synthesis but it has been used for the mutation process in the DNA.
References:
- G. Buchi, C. G. Inman, E. S. Lipinsky, ¨ J. Am. Chem. Soc. 1954, 76, 4327–4331
- Laue T.,Plagens A.,Named Organic Reactions, Second edition, John Wiley & Sons, Ltd, 2005
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010






