Table of Contents
ToggleIn the Norrish type II reaction, the photochemical intramolecular abstraction of γ-hydrogen (a hydrogen atom three-carbon positions apart from the carbonyl group) by the excited carbonyl compound forms a 1,4-biradical as a major photoproduct. Norrish first reported the reaction in 1937. The resulting biradical undergoes secondary reactions such as fragmentation to form an enol and an alkene or undergo intramolecular recombination of the two radicals to a substituted cyclobutane (the Norrish-yang reaction).
Primary process
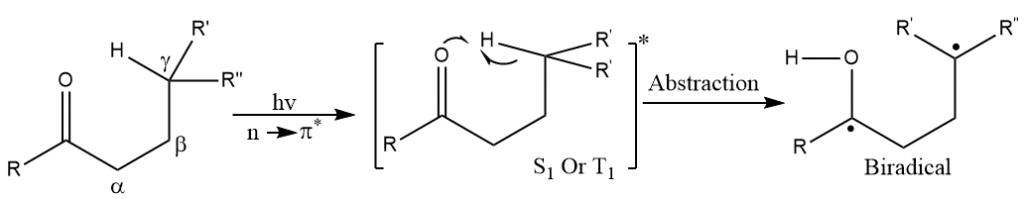
Absorption of light in the range of 230-330nm causes n to π* excitation to form an excited singlet state an excited triplet state by ISC (Inter System Crossing).
Secondary process
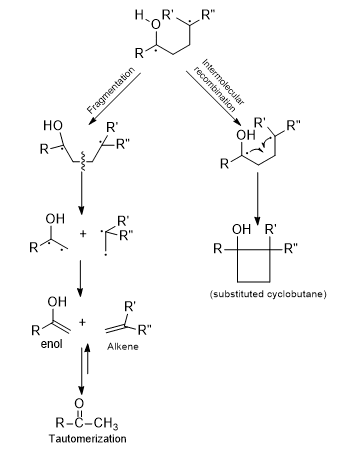
Depending upon the conformation of initially formed 1,4-diradical two different pathways are possible to stabilization.
If only the sp-orbitals of the radical centers could overlap, a cyclobutane would be the product. If the sp-orbitals of the radical centers are parallel to the β-bond, they participate in the formation of two double bonds (one in the enol and another in the alkene) as a result of the cleavage of the β-bonds.
Scope of Norris II reaction
•An example of a synthetically useful Norrish type II reaction can be found early in the total synthesis of the biologically active cardenolide ouabagenin by Baran and coworkers.
•The optimized conditions minimize side reactions, such as the competing Norrish type I pathway, and furnish the desired intermediate in good yield on a multi-gram scale.
References
- Handbook of Synthetic Photochemistry: Albini, Angelo, Fagnoni, Maurizio: Books. (n.d.). Retrieved March 4, 2022
- Applied Photochemistry: Evans, Rachel C., Douglas, Peter, Burrow, Hugh D.
- Kalaivani, s. (2013). Organic Photochemistry and Pericyclic Reactions (1st edition). MJP Publishers.






