Table of Contents
ToggleUgi reaction mechanism, examples, and applications in organic chemistry are discussed here.
Ugi reaction
Ugi reaction is a four-component condensation reaction of an amine, a carbonyl compound, a carboxylic acid, and an isonitrile that forms an α-acylamino amide. This reaction is also known as Ugi condensation, Ugi multicomponent condensation, Ugi multiple-component condensation, or Ugi four-component condensation. Ugi reaction is closely related to passerini reaction.
The overall reaction is essentially a domino reaction involving numerous subreactions. The Ugi reaction is usually exothermic, and carried out at room temperature in methanol, giving 1:1 syn/anti diastereoisomers on the newly generated asymmetric center.
Ugi reaction can be presented as:
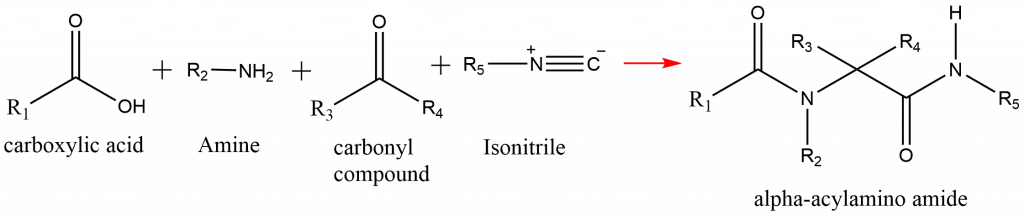
Ugi reaction mechanism
Ugi reaction proceeds with an initial condensation reaction between an amine and a carbonyl compound that form an imine via the loss. The protonation of the imine by a carboxylic acid, and the further reaction of the iminium ion with isonitrile and the carboxylate leads to the formation of Ugi product, α-acylamino amide.
The mechanism take place by the following way:
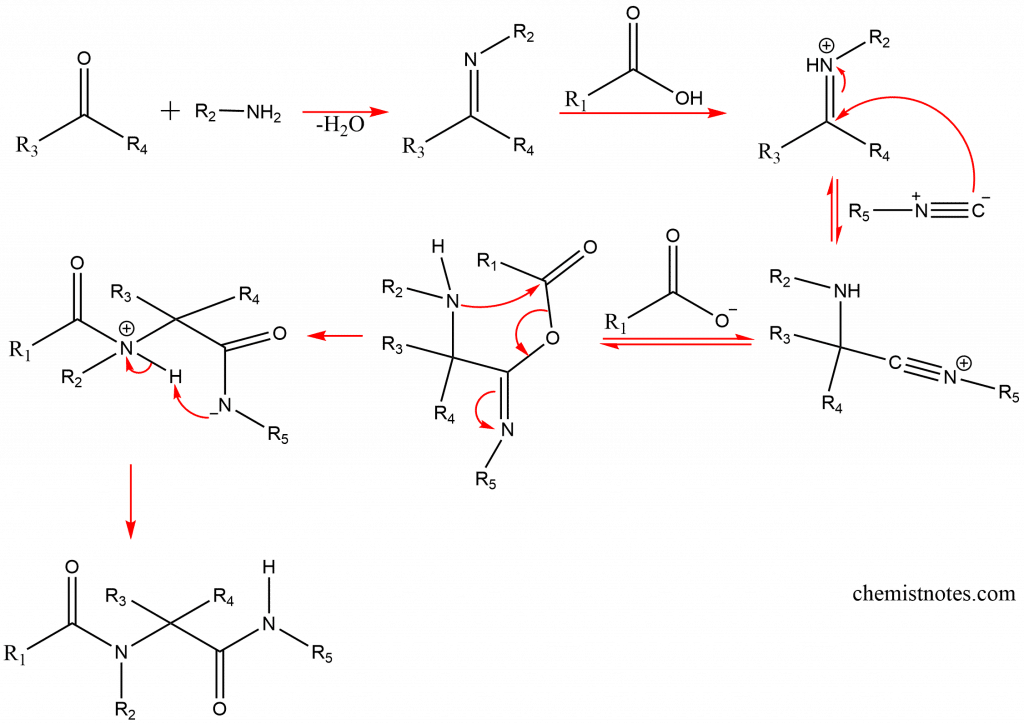
Application of Ugi reaction
This reaction has a wide range of applications in organic synthesis, particularly in the generation of structurally diverse molecular libraries by using combinatorial synthesis. Some of the drugs like Crixivan, bupivacaine has been also synthesized, and hence Ugi reaction have significant uses in the pharmaceutical industry.
Ugi reaction Video
FAQs/MCQs
What is Ugi reaction?
Ugi reaction is the multicomponent reaction of an amine, a carbonyl compound, a carboxylic acid, and an isonitrile that yields an α-acylamino amide.
References
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
- Williams, D. R.; Walsh, M. J. Ugi Reaction. In Name Reactions for Homologations-Part II; Li, J. J., Corey, E. J., Eds.; Wiley & Sons: Hoboken, NJ, 2009, pp 786−805. (Review).
- Ugi, I.; Meyr, R.; Fetzer, U. and Steinbruckner, C., ¨ Angew. Chem., 1959, 71, 386. (b) Ugi, I.and Steinbruckner, C., ¨ German Patent, 1,103,337, 1959.
- Endo, A.; Yanagisawa, A.; Abe, M.; Tohma, S.; Kan, T.; Fukuyama, T. J. Am. Chem.Soc. 2002, 124, 6552−6554.