Table of Contents
ToggleIn this blog, we will try to explain the oxymercuration-demercuration reaction and its mechanism by comparison with acid-catalyzed hydration of alkene.
Introduction to acid-catalyzed hydration of alkene
In acid-catalyzed hydration of alkene, the Markovnikov rule is followed so that the hydrogen from the water molecule is attached to that part of the double bond having more hydrogens. So, from this consideration, we might expect only one product.
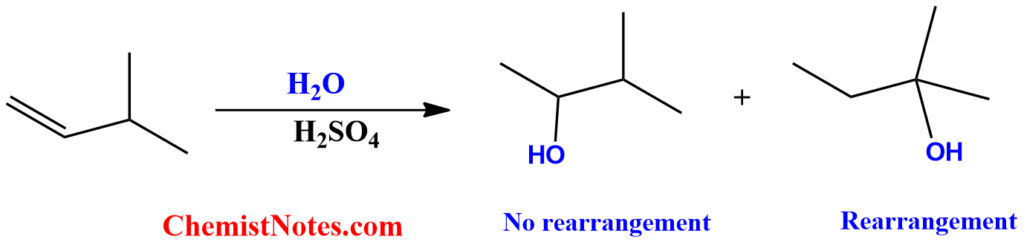
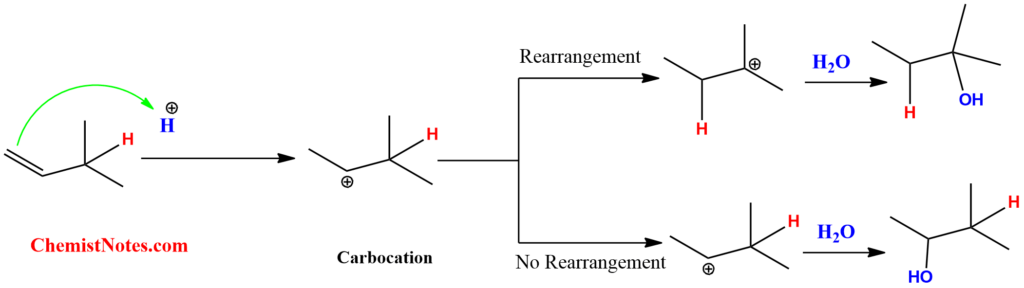
However, this is not true. Due to carbocation rearrangement, there is the formation of a mixture of products. We can understand this by looking at the following example.
Oxymercuration-demercuration reaction
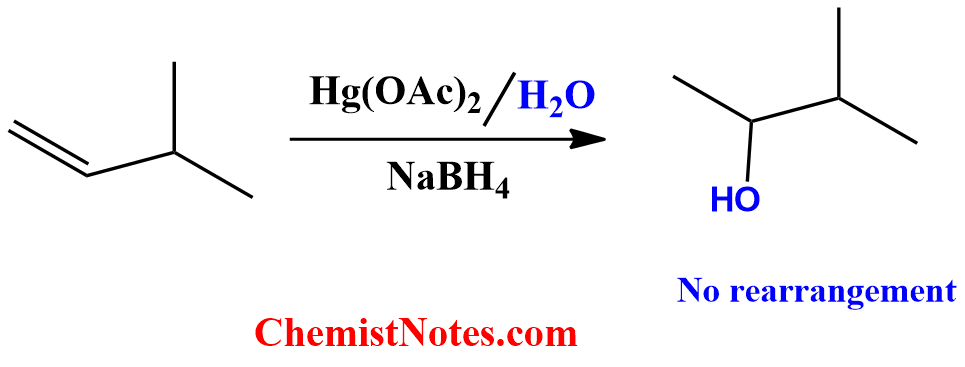
To avoid the above-mentioned phenomenon where protonation of the double bonds in alkene ultimately leads to the carbocation rearrangement, there is one reaction known as oxymercuration-demercuration.
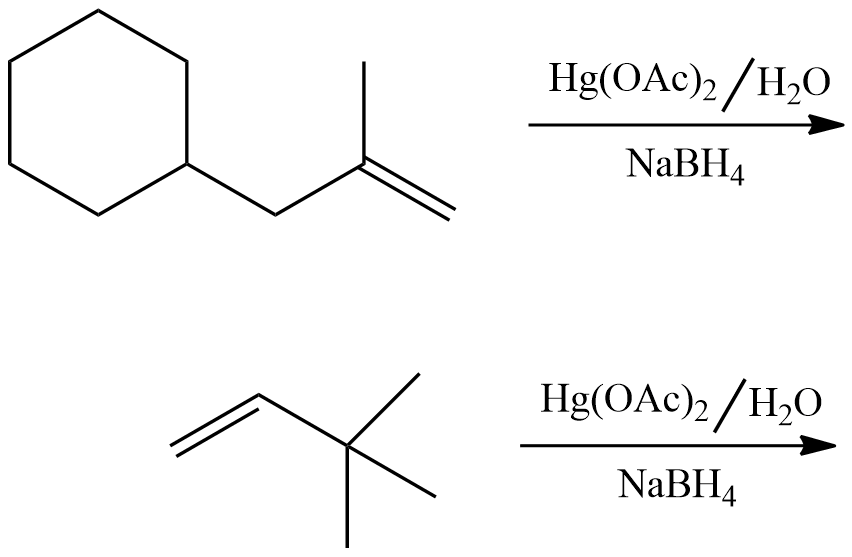
Oxymercuration-demarcuration reaction involves Markovnikov’s addition of water across an alkene without carbocation rearrangement by using mercuric acetate and sodium borohydride reagents.
Oxymercuration-demercuration reaction mechanism
This reaction includes two steps: oxymercuration and demercuration.
Step 1: In the first step, the mercuric acetate Hg(OAc)2 dissociates, forming a mercuric cation, which is a strong electrophile, and hence it is attacked by the pie-electrons of the alkene. The resulting intermediate is known as a mercurinium ion. This process is called mercuration.
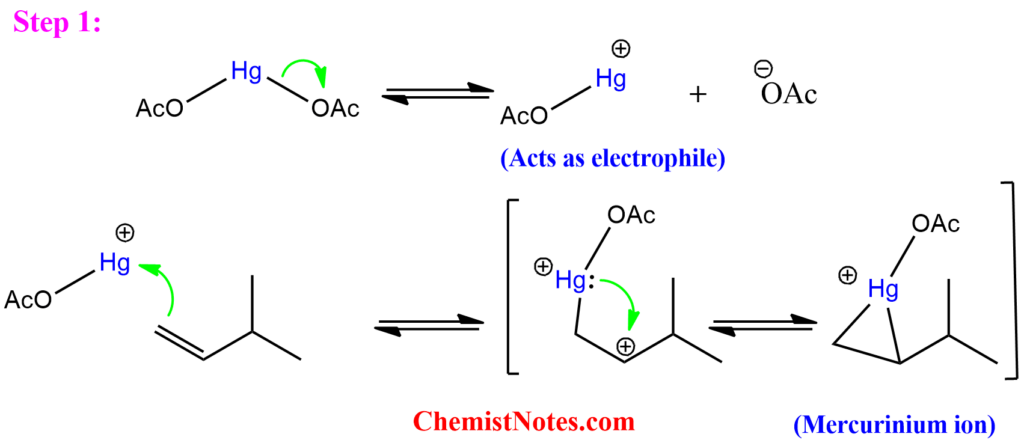
Step 2: In the second step, the nucleophile (usually H2O) attacks the mercurinium ion at the more substituted position, leading to Markovnikov addition. Finally, the mercury is removed by adding sodium borohydride. This process is known as demercuration.
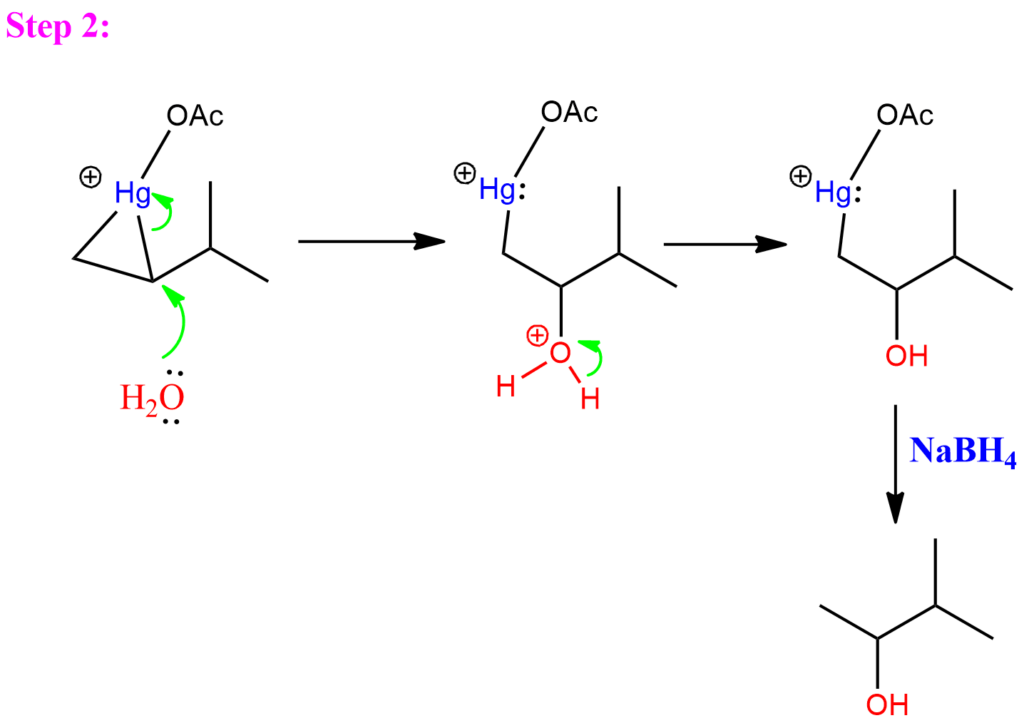
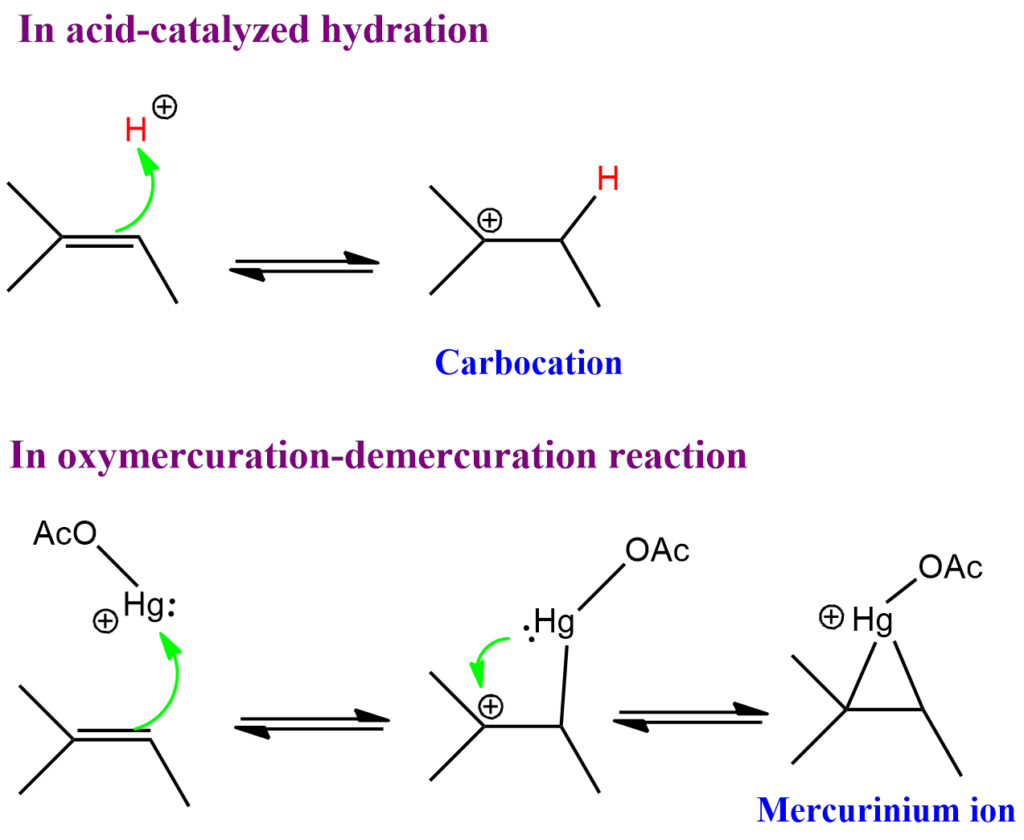
Acid-catalyzed hydration Vs Oxymercuration-demercuration reaction
The major difference between acid-catalyzed hydration and oxymercuration demercuration reactions is that carbocation is formed as an intermediate in acid-catalyzed hydration, whereas mercurinium ion is formed as intermediates in oxymercuration demercuration reaction. Moreover, there is a high probability of rearrangement of the carbocation formed in the first reaction. However, there is no such rearrangement taking place in mercurinium ion.
Oxymercuration-demercuration reaction of 1-methylcyclohexene
Oxymercuration-demercuration reaction of 1-methylcyclohexene gives 1-methylcyclohexanol and the mechanism is the same as described above.
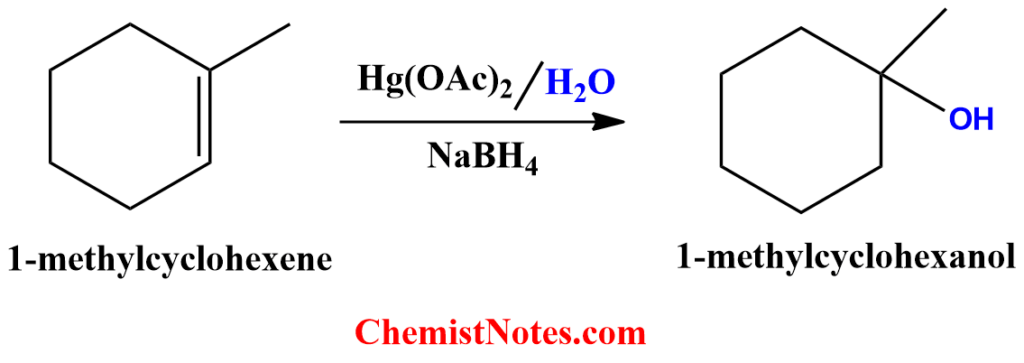
Solve the following problems: