Table of Contents
ToggleThe term “nucleophilic substitution reaction” refers to a type of organic reaction in which one nucleophile substitutes another. It is quite similar to the typical displacement reactions seen in chemistry, in which a more reactive element replaces a less reactive element in its salt solution.
Allylic halogenation
Alkenes can be brominated in the allylic position and also in a benzylic position by a number of reagents, of which N-Bromo succinamide (NBS) is by far the most common. If bromination can be done by using this reagent, then the reaction is called Wohl–Ziegler bromination. The process is carried out in an ionic liquid using a nonpolar solvent, most often CCl4. With any reagent, an initiator is needed; this is usually a peroxide (e.g., di-tert-butyl peroxide) or benzoyl peroxide, or less often, UV light.
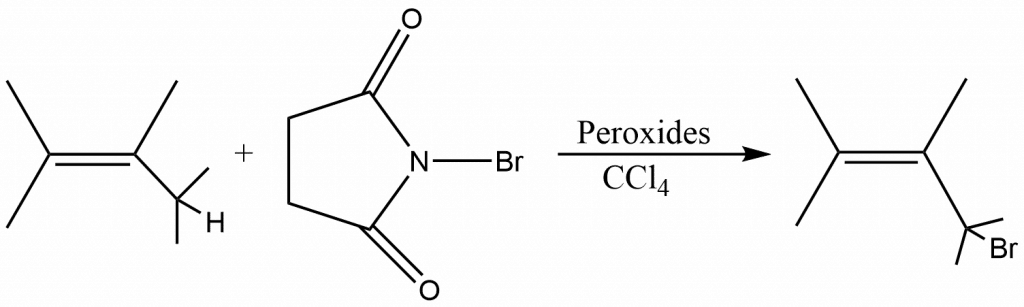
At an allylic or benzylic position, the reaction is usually quite specific, and high yields are produced. However, when the allylic radical intermediate is unsymmetrical, allylic rearrangements can occur, leading to combinations of both potential products.
Allylic Bromination using NBS
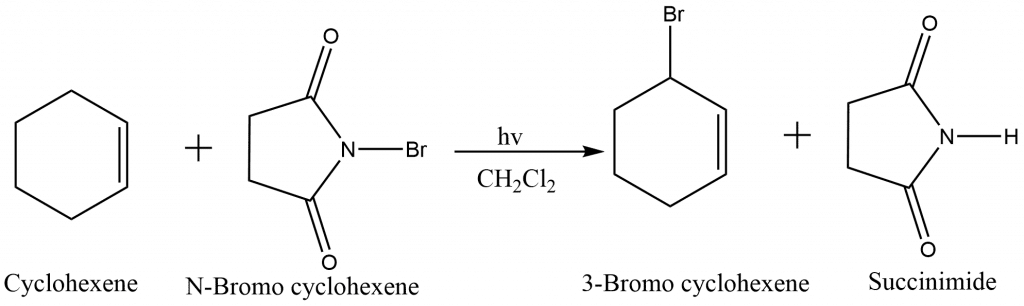
N-Bromosuccinimide is a highly regioselective brominating agent at other positions, including positions α to a carbonyl group, to a C≡C triple bond, and to an aromatic ring (benzylic position). When both a double and a triple bond are present in the same molecule, the preferred position is α to the triple bond. The free radical mechanism of allylic bromination demonstrates that the reaction is extremely sensitive to free radical initiators and inhibitors, and that it will not proceed at all unless at least a trace of initiator is present.The reaction is initiated by small amounts of Br•.

Refrences






