Table of Contents
ToggleMetal hydride reduction, examples, mechanisms, and applications in organic chemistry have been discussed here:
Metal hydride reduction Definition:
Aldehydes and ketones are converted to primary and secondary alcohols by metallic hydrides such as lithium aluminum hydride (LiAlH4) and sodium borohydride (NaBH4). Such a reaction is called metal hydride reduction reaction. The hydride ion (H–) acts as a nucleophile in this case. By hydride transfer, these metallic hydrides are also used to reduce acid halides, carboxylic acids, esters, amides, and other compounds.
Typically, these metallic hydrides do not reduce carbon-carbon double or triple bonds. However, LiAlH4 reduces carbon-carbon multiple bonds in conjugation with an aromatic system on one side of a carbonyl group on the other side.
Some of the examples of metal hydride reactions are

Metal hydride reduction mechanism
The mechanism of metal hydride reduction can be carried out in the following 2 steps:
Step 1: During nucleophilic addition, lithium aluminum hydride (LiAlH4) or sodium borohydride (NaBH4) provides hydride ions (H–) as a nucleophile that attacks the electron-deficient carbonyl carbon.

Step 2: The excess LiAlH4 is carefully eliminated, and the aluminum salt of alcohol produced in step 1 is hydrolyzed by mineral acid to liberate the appropriate alcohol in a high yield.

Metal hydride reduction of an acid
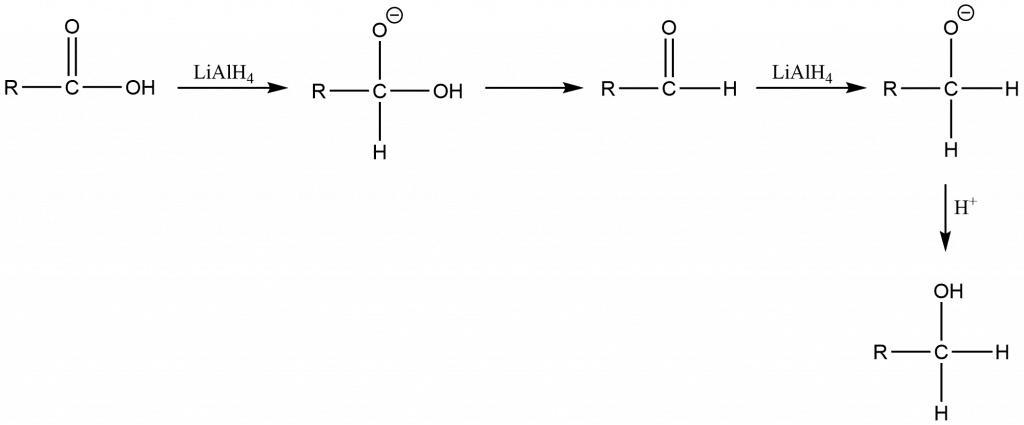
Metal hydride reduction of ester
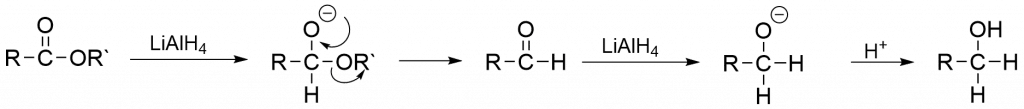
Metal hydride reduction of acid halide
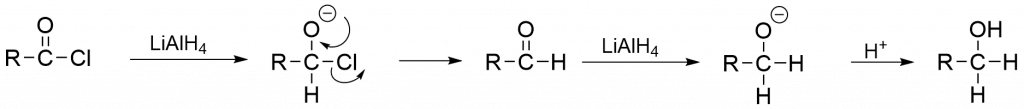
Application of Metal hydride reduction reaction
- Preparation of unsaturated alcohol from unsaturated carbonyl compounds
- Reduction of ester to alcohol
- Reduction of carboxylic acid to alcohol
- Reduction of acid halide to alcohol
Metal hydride reduction video
FAQs/MCQs
Metal hydride reduction of carbonyl compounds
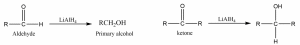
Carbonyl compounds are converted into primary or secondary alcohol when treated with metal hydride reagents ( LiAlH4 ) or ( NaBH4 ).
What is metal hydride reduction?
The reaction involving the reduction of a carbonyl compound (aldehyde or ketone) into corresponding primary or secondary alcohol when treated with metallic hydrides such as lithium aluminum hydride (LiAlH4) and sodium borohydride (NaBH4) called metal hydride reduction.
Example of metal hydride reduction
Aldehyde,s ketones, acid halides, carboxylic acids, esters, amides, including other compounds can be reduced into corresponding alcohol by metal hydride reduction reaction.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.