Table of Contents
ToggleLaboratory Preparation of ethyne/acetylene, its principle, procedure, and uses in organic chemistry have been discussed here:
Preparation of ethyne or higher alkynes
Some of the important methods of preparation of alkynes in the laboratory are discussed below:
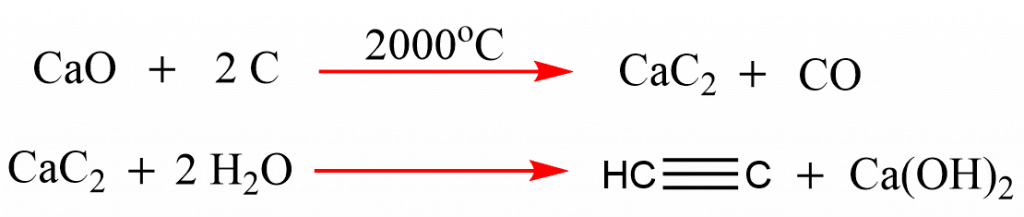
Preparation of ethyne from calcium carbide
When calcium carbide is treated with water, acetylene gas is produced.
Preparation from carbon and hydrogen
Acetylene is synthesized by passing a stream of hydrogen through an electric arc between carbon electrodes. This preparation process is performed at 3000oC.

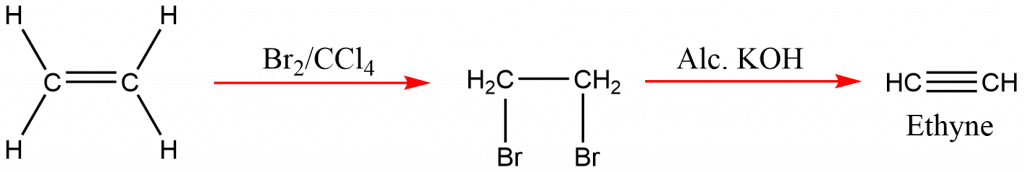
Preparation of ethyne from ethene
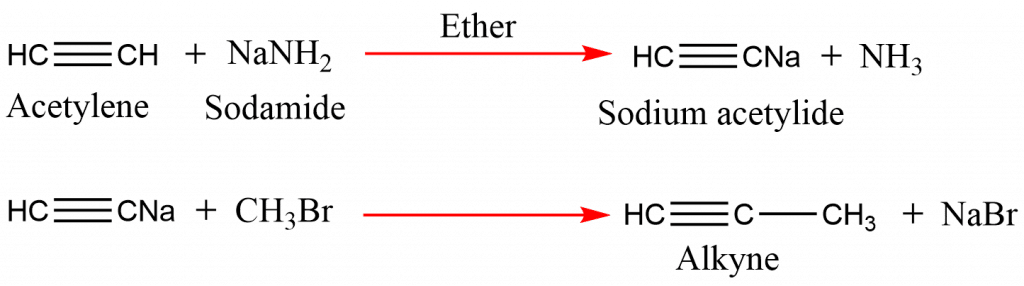
Synthesis from acetylene
On treating sodium salt with alkyl halides, higher alkyne can be synthesized from acetylene.
From dehydrohalogenation of dihalides
When vicinal dihalides are treated with an alcoholic solution of KOH, then acetylene/higher alkynes are prepared by a dehydrohalogenation reaction.

From dehalogenation of dihalides
Alkynes are formed by dehalogenation reaction when tetrahalogen derivatives of alkanes are heated with zinc dust in ethanol.

Laboratory preparation of Acetylene
Following method is one of the major laboratory methods for manufacturing ethyne or acetylene in the laboratory.
Principle involved in laboratory preparation of acetylene
Acetylene can be prepared in the laboratory by treating calcium carbide with water.

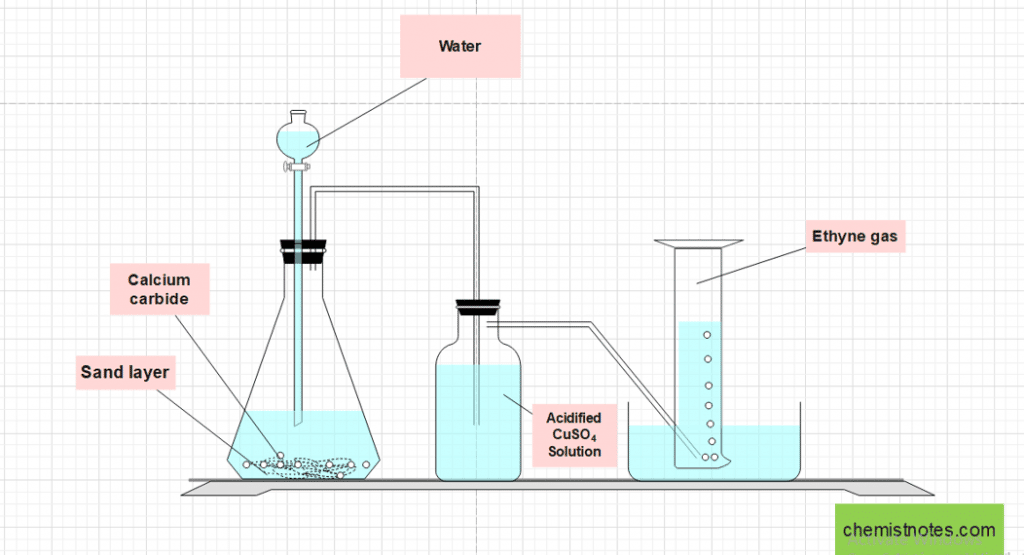
Procedure for the preparation of acetylene
Lumps of calcium carbide are placed on the layer of sand in a conical flask fitted with a dropping funnel and delivery tube. The free end of the delivery tube is inserted into a wash bottle containing acidified copper sulphate solution, and the wash bottle’s exit end is fitted into the beehive shelf, which is placed in a trough containing water.
When water is dropped into the flask, a violent reaction occurs, resulting in the formation of ethyne gas. The ethylene gas so produced contains impurities like H2S and PH3 and is eliminated by passing it through an acidified CuSO4 solution. Pure ethyne/acetylene gas is collected by the downward displacement of water.
Lab Preparation of Acetylene video
Uses of ethyne
- For the artificial ripening of fruits.
- Used to generate light.
- Used to prepare various organic compounds like ethanol, acetic acid, acetaldehyde, etc.
- For the manufacture of plastics, rubber, synthetic fibers, and so on.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice-Hall, London, 1995.






