Table of Contents
ToggleLaboratory Preparation of ethene/ethylene, its principle, procedure, and uses in organic chemistry have been discussed here:
Preparation of Ethene
Some of the important methods of preparation of alkenes in the laboratory are discussed below:
From alkyl halides
When alkyl halides are treated with alcoholic KOH, a HX (hydrogen halide) is removed, resulting in the formation of alkenes. This is a dehydrohalogenation reaction.
Here’s one of the examples of the preparation of ethene from ethyl bromide:

From dihalogen derivatives
Dihalogen derivatives such as vicinal dihalides can be converted into alkenes by heating with zinc dust in ethanol.

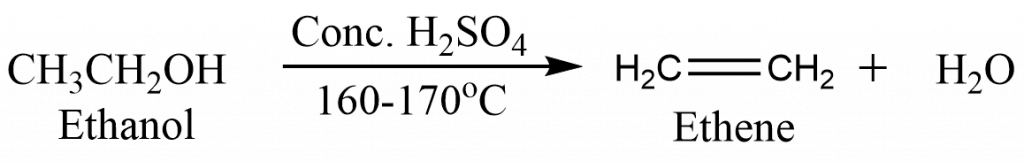
From alcohol
Alcohols are dehydrated when heated in concentrated sulphuric acid, yielding alkenes.
From partial hydrogenation of alkyne
On passing hydrogen over alkynes in the presence of a mixture of palladium and Barium sulphate (BaSO4) at around 200oC, alkene can be obtained.
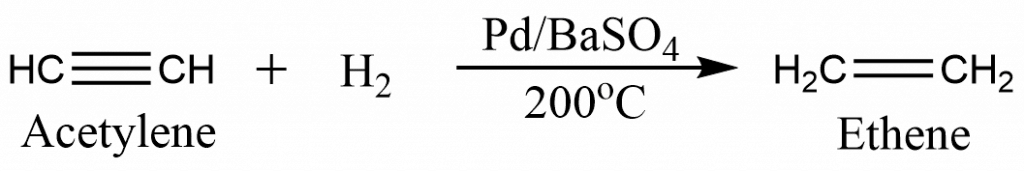
Laboratory preparation of Ethene
Following method is one of the major laboratory methods of manufacturing ethene in the laboratory.
Principle involved in laboratory preparation of ethene
Ethene/Ethylene gas can be prepared in the laboratory by heating ethyl alcohol with sulphuric acid at about 160-170oC.

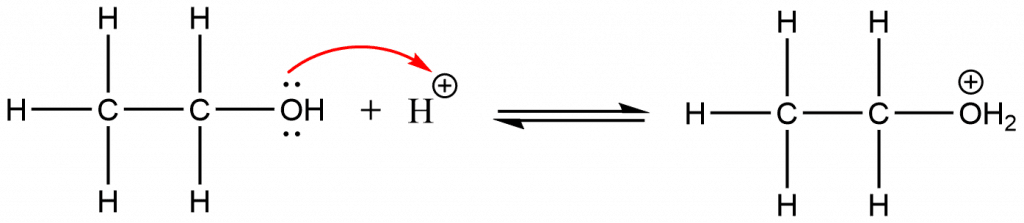
The mechanism of the above dehydration reaction takes place as:
Step 1: Protonation of alcohol
Step 2: Formation of carbocation; loss of water molecule
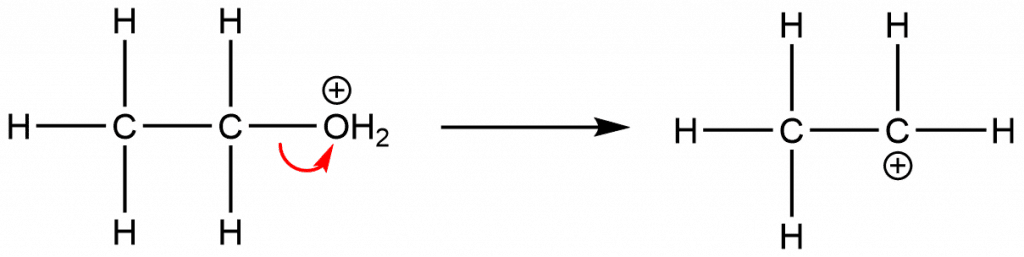
Step 3: Formation of alkene via the loss of a proton from generated carbocation

Procedure for the preparation of ethene
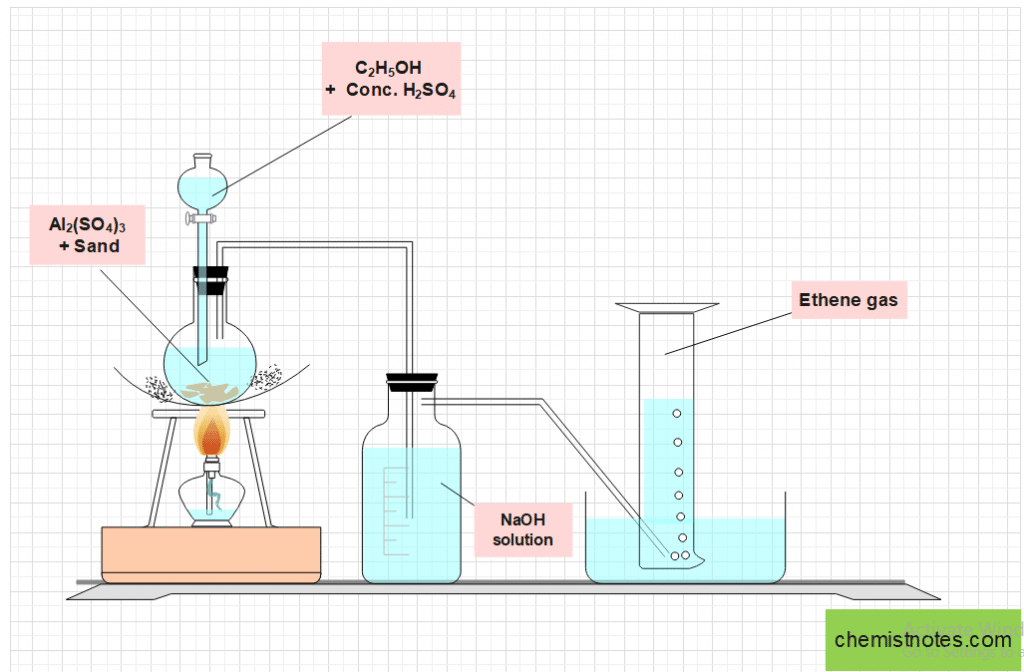
A round bottom flask fitted with a dropping funnel and a delivery tube is filled with a 1:2 mixture of ethyl alcohol and concentrated sulphuric acid. A small amount of aluminum sulphate and sand are also added to facilitate dehydration and prevent frothing. Now, the delivery tube’s free end is inserted into a wash bottle containing sodium hydroxide solution, and the wash bottle’s exit end is fitted into the beehive shelf, which is set in a water-filled trough.
Ethylene gas is produced when the flask is heated at 160-170oC. The impurities like CO2 and SO2 are present in the ethylene produced and are removed using NaOH solution. Pure ethylene gas can be collected by downward displacement of water.
Lab Preparation of ethene video
Uses of ethene
- For the artificial ripening of fruits
- Used as an anesthetic in hospitals
- For the manufacture of glycol, ethanol, ethyl halide, etc.
- For the manufacture of polyethylene
- Used as a solvent in cosmetics, pharmaceutics, and so on.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and Company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice-Hall, London, 1995.






