Table of Contents
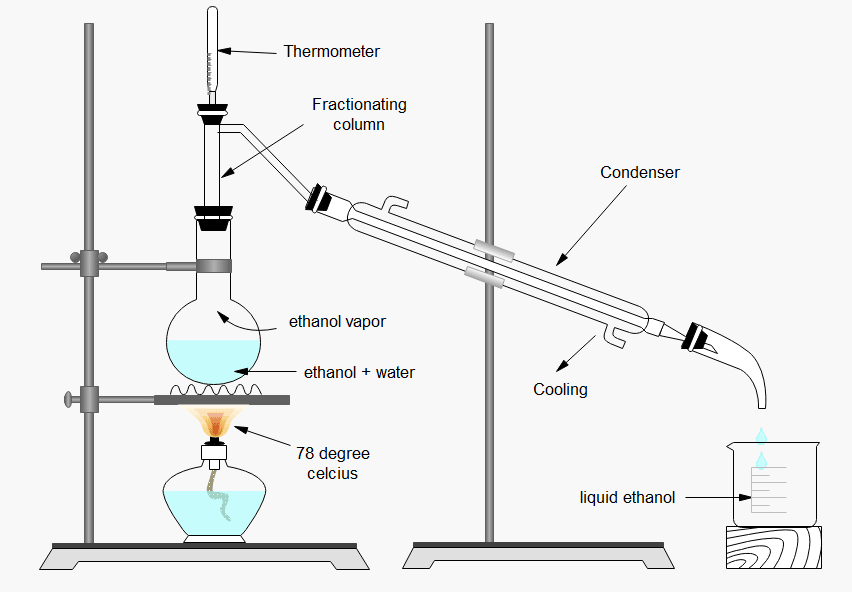
ToggleIn this post, we are going to discuss the Fractional distillation of ethanol and water, Apparatus setup, and Process of fractional distillation of alcohol. Since both the liquids are volatile, miscible with each other, and have a difference in their boiling point, ethanol can be separated from a mixture of ethanol and water via fractional distillation. When the mixture is heated in a distillation flask, ethanol which has a low boiling point (78oC) than water (100oC) boils faster. Hence ethanol vapor passes into the condenser (the region where vapor is cooled and condensed), collected in a beaker, and hence ethanol gets separated from the mixture. In the cases where most of the ethanol is left out, water vapors at 100oC and condenses. In this way, the separation of liquid from its mixture takes place.
Process for fractional distillation of ethanol and water
The process of fractional distillation of ethanol and water is discussed below:
- Load the round-bottom flask with the ethanol-water mixture, then assemble the fractional distillation apparatus by connecting the fractionating column to the flask.
- To capture the distillate, connect the condenser to the fractioning column and put the distillate-capturing flask underneath it.
- Heat the mixture to above the boiling point of ethanol (80 degree celcius) using the Bunsen burner under the round-bottom flask.
- Maintain a consistent temperature in the mixture until the boiling has stopped. You’ve finished distillation at this point.
Apparatus of fractional distillation of alcohol
FAQs
Is fractional distillation a physical or chemical change?
In fractional distillation, separation of a mixture of miscible liquid is carried out by means of heating where the change in the state ( liquid to vapor) takes place without any change in its chemical composition. Thus, Fractional distillation possesses physical change
Separation of ethanol and water
As both the liquids are miscible and have low differences in their boiling point, separation of ethanol and water can be carried out by fractional distillation
Fractional distillation example
Separation of mixture of ethanol and water is one of the examples of fractional distillation






