Table of Contents
ToggleVictor Meyer’s method is a quantitative approach for determining the molecular mass/weight of volatile compounds. The molecular weight of a substance is the average relative weight of one molecule of it as compared (1/12)th of the weight carbon (C-12) taken as 12 amu. Some of the methods that involve the determination of the molecular weight of compounds are:
- Physical methods: elevation in boiling point, depression in freezing point, Victor Meyer’s method
- Chemical methods: Volumetric method, silver salt method
Victor Meyer’s Method
A known amount of the substance is converted into vapours using this method, and the volume of the vapours produced is collected over water and measured at laboratory temperature and pressure. After converting the volume to NTP, the weight of the same volume of hydrogen is calculated. The molecular weight of a substance is obtained by calculating the vapour density of the substance.
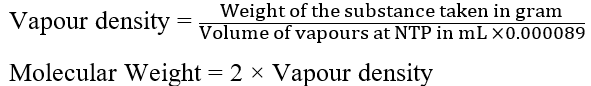
Determination of molecular mass by Victor Meyer’s method: Procedure
The apparatus employed in Victor Meyer’s method consists of a tube, also known as Victor Meyer’s tube whose upper end is funnel-shaped, and the lower end is cylindrical bulb-like. The upper end is enclosed by a rubber stopper, the lower end in a heating jacket, and the side tube into a beehive shelf, which is set in a water-filled trough. [Note: The bath liquid in the heating jacket must have a 20-30oC higher boiling point than the substances under study].
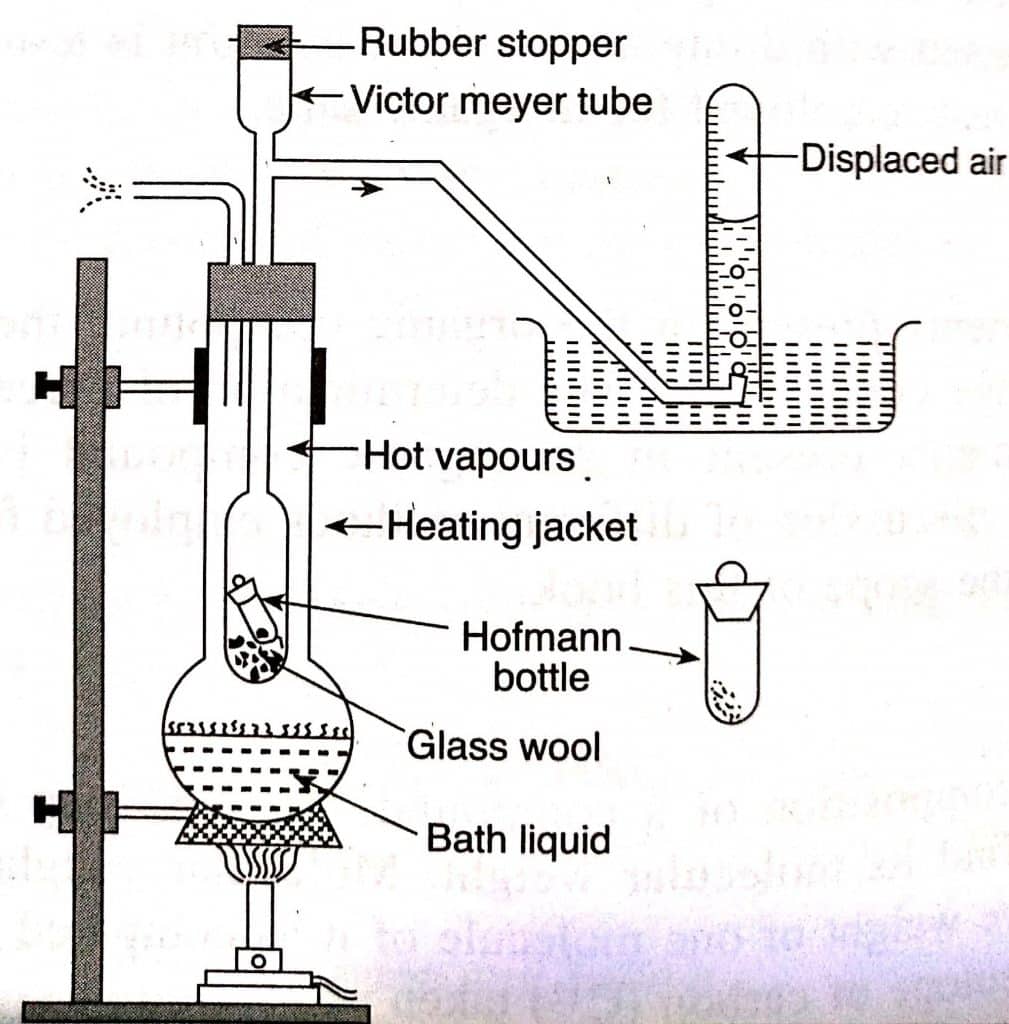
Hofmann’s bottle is filled with a small amount of the substance, and the liquid from the heating jacket is boiled. Some of the air inside Victor Meyer’s tube is forced out, and it bubbles to the surface. Only if there is no bubbling, a eudiometer tube full of water inverted over the beehive shelf. The rubber stopper is now removed from Hoffman’s bottle, and it is inserted into Victor Meyer’s tube. The volatile compounds in the bottle are transformed to vapours when it reaches the bottom, blowing off the stopper and displacing an equal volume of air from Victor Meyer’s tube.
The displaced air is then collected in a eudiometer tube by downward displacement of water. A eudiometer tube is immersed in a jar full of water, and the pressures are equalised. The parameters like the volume of displaced air, atmospheric pressure, and aqueous tension are noted.
Calculation of molecular mass
Let, the volume of vapours collected over water = V cc
Weight of the substance taken = w g
Temperature of water = T K
Atmospheric pressure = p mmHg
Aqueous tension at T K = f mmHg
Thus, pressure of dry air (P) = (p-f) mmHg
Using the gas equation, the volume is converted to NTP as [Note: P1 = 760 mmHg, T1 = 273 K, V1 =?]
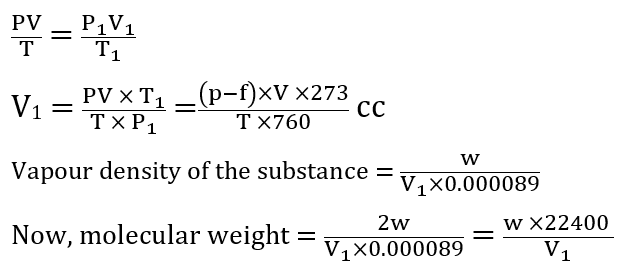
Thus, the molecular mass of a substance can be determined via Victor Meyer’s method.
Applications of Victor Meyer’s Method
- Determination of molecular formula as well as the empirical formula.
- Determination of vapour density of volatile compounds.
- Identification of Alcohols
Victor Meyer’s Method Video
FAQs/MCQ
Empirical formula
Empirical formula is the simplest form of expressing the elemental composition of a compound.
Molecular Formula
Molecular formula is the actual representation of the elemental composition of the compound.
Can Victor Meyer’s method identify alcohol?
Yes, Victor Meyer’s method is used to identify Primary (1o), Secondary (2o) and Tertiary(3o) alcohols.
Vapour density
The density of vapour in relation to the density of hydrogen vapour is known as vapour density.
References
- General Chemistry-John Russell by McGraw Hill International Editions 3rd edition.
- Intermediate Chemistry, Inorganic and Physical. Prescott (1965).
- University General Chemistry-An Introduction to Chemical Science edited by CNR Rao by McMillan Indian Ltd.