Table of Contents
ToggleLaboratory Preparation of chloroform or trichloromethane, its principle, procedure, purification process, and uses in organic chemistry have been discussed here:
Principle involved in laboratory preparation of chloroform
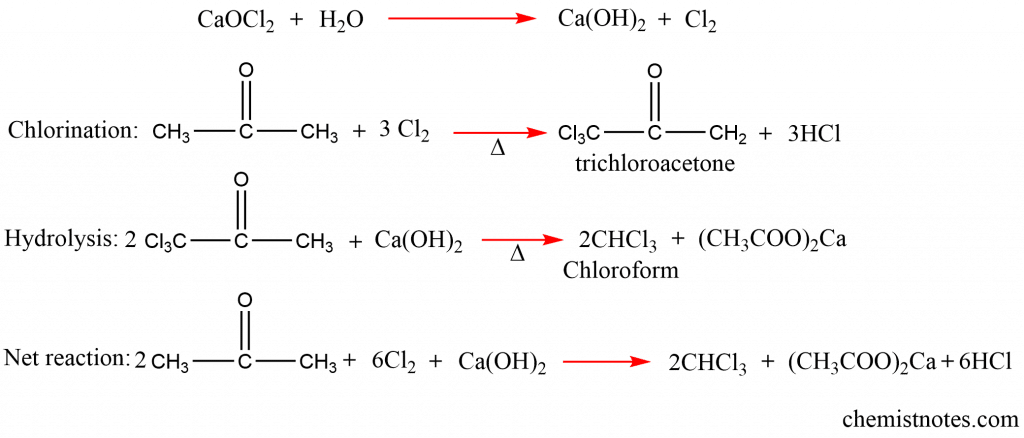
Chloroform is prepared in a lab by heating ethyl alcohol or acetone with bleaching powder paste. Bleaching powder has the ability to oxidize, chlorinate, and hydrolyze.
Chloroform can be obtained from ethanol as:

From acetone, preparation of trichloromethane can be done as:
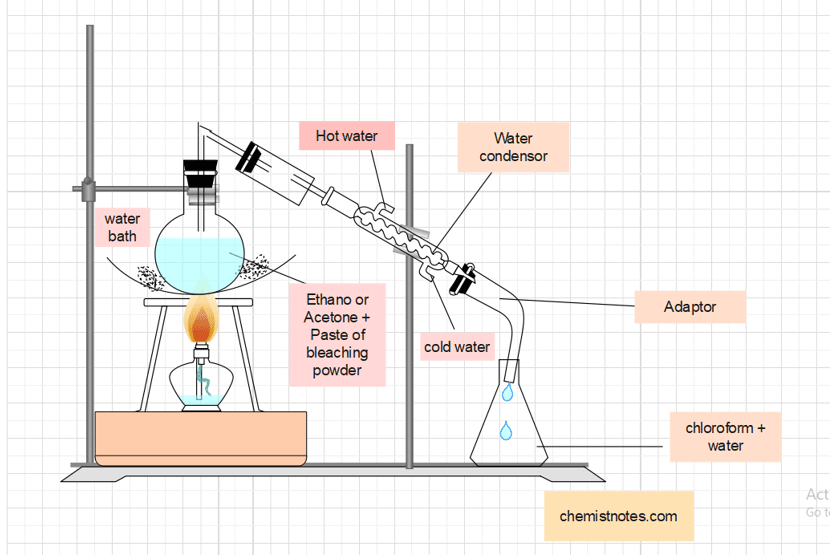
Procedure for the preparation of chloroform
In an R.B. flask fitted with a water condenser, a mixture of ethyl alcohol or acetone (25 mL) and bleaching powder (100g) paste in 200 mL water is taken. The mixture is then gradually heated over a water bath until a mixture of chloroform and water is distilled over. The lower layer of chloroform is separated from the receiver using a separating funnel.
Purification of chloroform
The chloroform thus obtained is impure, hence first treated with dilute caustic soda (NaOH) solution to remove acid and then washed with water successively in the separating funnel. It is then dried over anhydrous calcium chloride and redistillation is performed at 60 – 650C to obtain pure and dry chloroform.
Lab Preparation of chloroform video
Uses of chloroform
- Used for the preparation of salicylaldehyde, chloropicrin, chloretone, etc.
- Used as a preservative for anatomical specimens.
- Used as a solvent for floor polishes, resins, adhesives, fats, oils, waxes and rubber.
- Used as an intermediate in the manufacture of polymeric materials.
- Used as an anesthesia, pain killer, and used as a remedy for cough.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.






