Table of Contents
ToggleLaboratory Preparation of formic acid (methanoic acid), anhydrous formic acid, and its uses in organic chemistry have been discussed here:
Principle involved in laboratory preparation of Formic acid
In the laboratory, formic acid is prepared by heating oxalic acid with anhydrous glycerol at about 100-110oC. In other words, methanoic acid can be obtained by the decarboxylation of oxalic acid with glycerol at 1100C.
The reaction completes in the following three steps:
Step 1: Formation of glycerol mono-oxalate
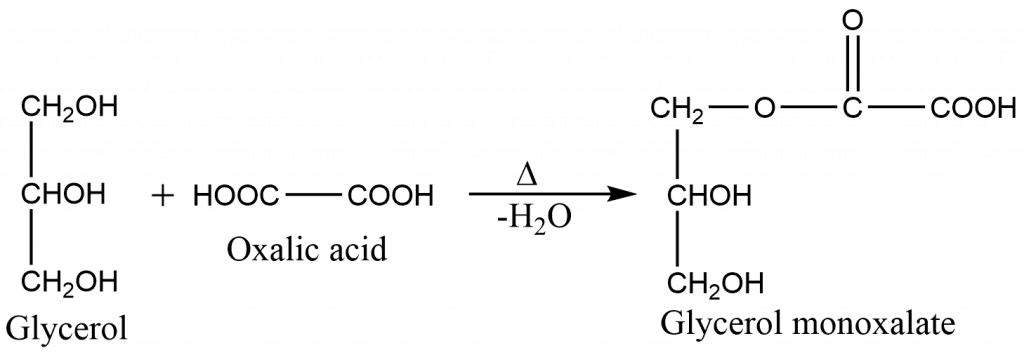
Step 2: Formation of glycerol monoformate
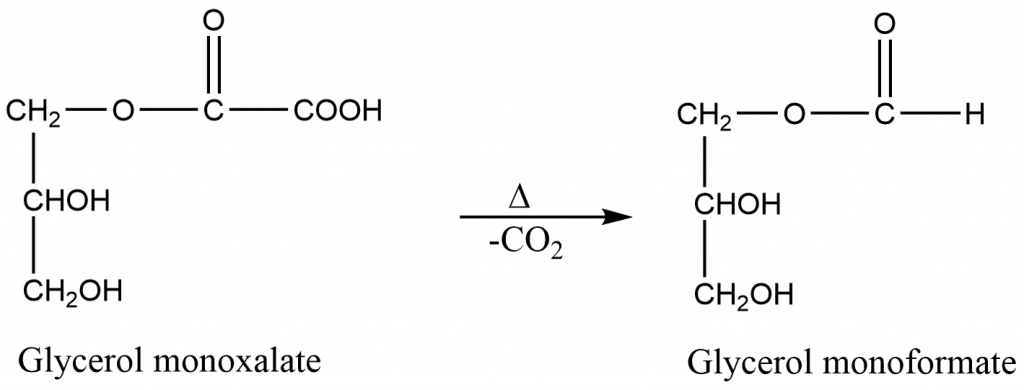
Step 3: Formation of formic acid
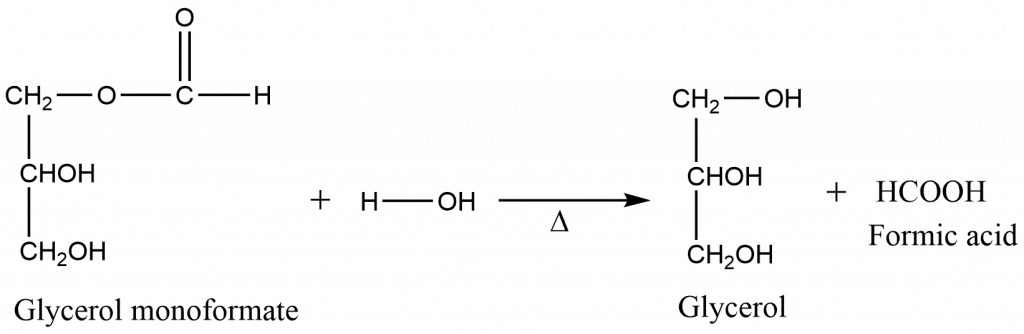
Procedure for the preparation of formic acid from oxalic acid
In a distillation flask fitted with a thermometer and condenser, 40 grams of powdered oxalic acid and 50 mL of anhydrous glycerol are taken. Heat the flask to around 110°C until the effervescence ceases. After cooling the flask, a fresh lot of 40-gram oxalic acid is added. The mixture is then heated at 110°C, after which an aqueous formic acid solution is collected in the receiver.
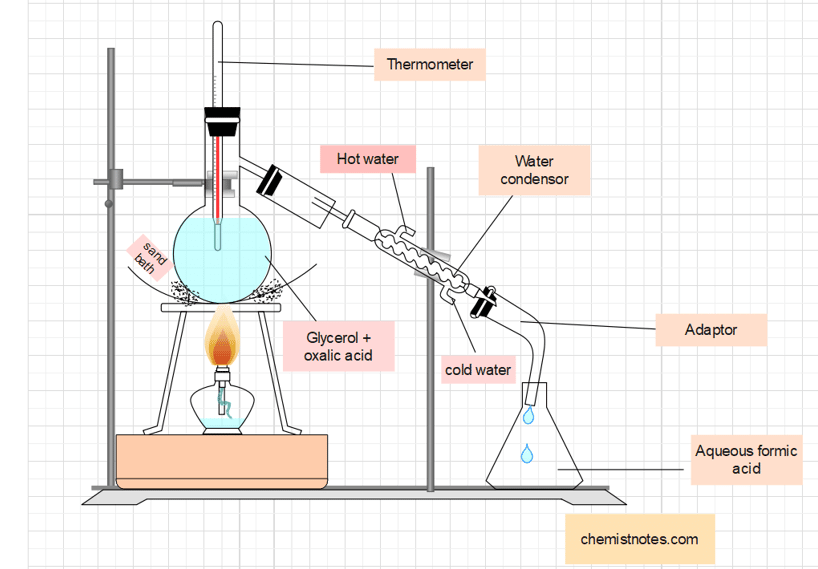
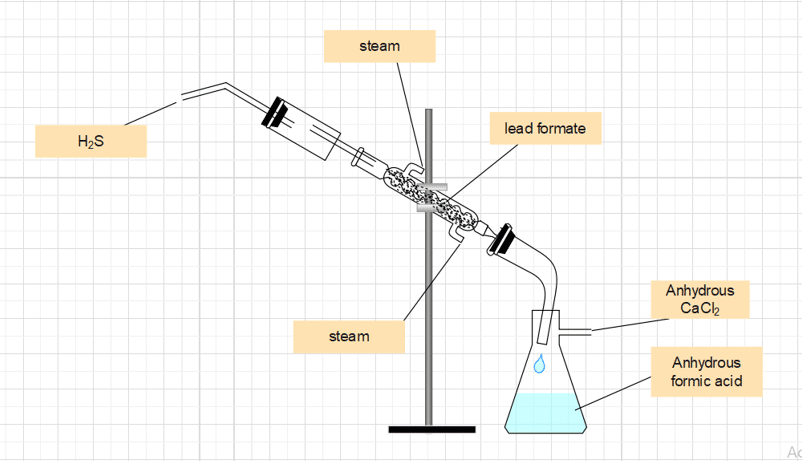
Laboratory preparation of anhydrous formic acid
The chemical method is applied to get Anhydrous formic acid as it cannot be obtained from aqueous formic acid even by fractional distillation, as the boiling point of formic acid (100.5oC) is the same as that of water.
To obtain crystals of lead formate, the aqueous formic acid is neutralized with lead carbonate, and the lead formate solution is evaporated. The inner tube of the water condenser is filled with dry lead formate, which is then treated with dry H2S gas. Anhydrous formic acid is produced as a result, and it is collected in the receiver. There are traces of H2S in this anhydrous formic acid. To obtain pure formic acid, it is combined with some lead formate and distilled.

Lab Preparation of formic acid video
Uses of formic acid
- For the preservation of foods.
- Antibacterial agent in livestock feed
- For the laboratory preparatiion of carbon monoxide.
- As a coagulating agent in rubber industry.
References
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987.
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Bahl, B.S., A., Advanced Organic Chemistry, S. Chand and company Ltd, New Delhi, 1992.
- Finar, I. L., Organic Chemistry, Vol. I and Vol. II, Prentice Hall, London, 1995.






