Table of Contents
ToggleJulia olefination reaction, mechanism, examples, and applications in organic chemistry are discussed here:
Julia Olefination Reaction
Aldehyde or ketone reacts with sulfones in the presence of a base to produce an adduct which on treatment with acetic anhydride gives acetyl derivative that undergoes reductive elimination in the presence of sodium amalgam to yield alkene. Such a reaction is called Julia Olefination reaction or Julia–Lythgoe olefination.
Julia Olefination reaction possesses high stereoselectivity for (E)-disubstituted alkene.
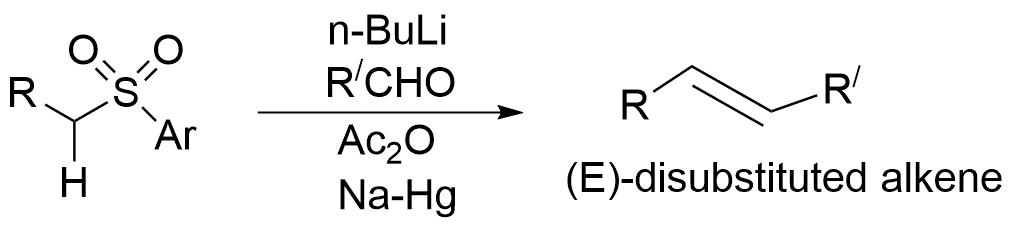
Julia Olefination Mechanism
Here, base abstracts Hydrogen and forms anion which reacts with an aldehyde to form the alkoxide. An adduct under treatment with acetic anhydride gives an acetyl derivative. Then, the reductive elimination takes place in presence of sodium amalgam and leads to the formation of (E)-disubstituted alkene.
The mechanism of Julia Olefination reaction can be carried out in the following way:
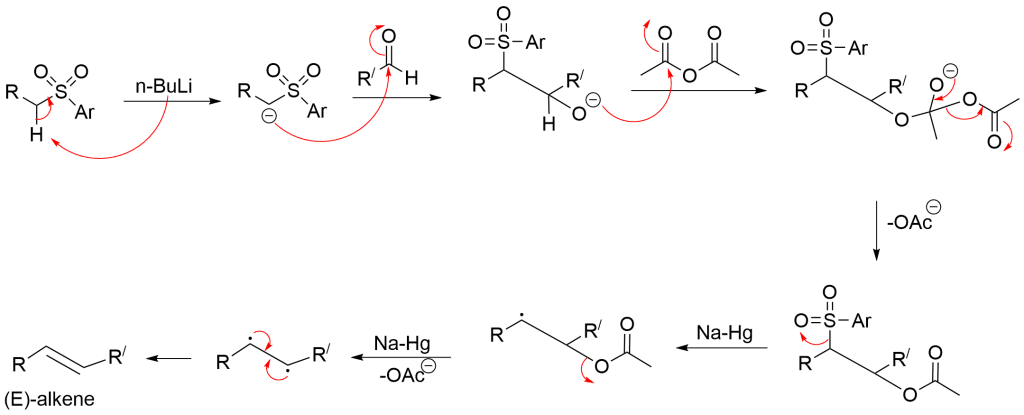
Applications of Julia Olefination reaction
Julia Olefination reaction has wide application in the preparation of alkene, particularly in the synthesis of complex natural products like Pterostilbene.
Julia Olefination reaction Video
FAQs/FAQs
Is Julia Olefination reaction also called Julia–Lythgoe olefination?
Yes, Julia Olefination is also known as Julia-Lythgoe olefination reaction.
What is Julia Olefination reaction?
The reaction that involves aldehyde or ketone with sulfone to yield highly stereoselective (E)-disubstituted alkene is called as Julia Olefination reaction.
References
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
- Julia, M.; Paris, J. M. Tetrahedron. Lett. 1973, 4833–4836. (b) Lythgoe, B. J.Chem. Soc., Perkin Trans. 1 1978, 834–837.
- Rong, F. Julia–Lythgoe olefination. In Name Reactions for Homologations-Part I; Li, J. J., Corey, E. J., Eds.; Wiley & Sons: Hoboken, NJ, 2009, pp 447−473. (Review).






