Table of Contents
ToggleFavorskii rearrangement reaction, examples, mechanism, quasi favorskii rearrangement and application have been discussed here. Favorskii rearrangement was first of all given by Favorski in 1894.
Favorskii rearrangement
Favorskii rearrangement is a base-catalyzed rearrangement of alpha-halo ketones to the carboxylic acid derivatives such as acids, esters, and amides. The product depends on the types of base used such as OH–,OR–, or NH2–. This reaction produces a product with the same number of carbon atoms in the skeletons.
Examples of favorskii rearrangement
Let’s see examples of Favorskii rearrangement reaction.
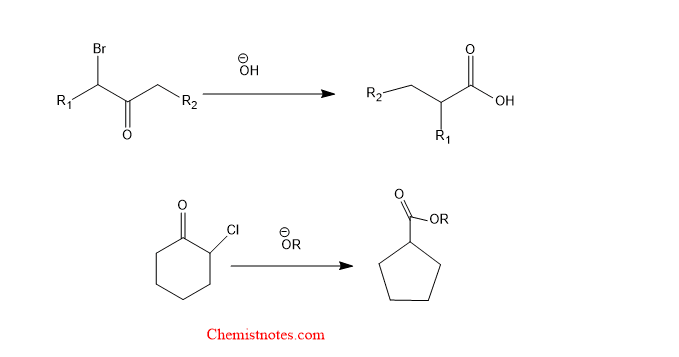
Favorskii rearrangement mechanism
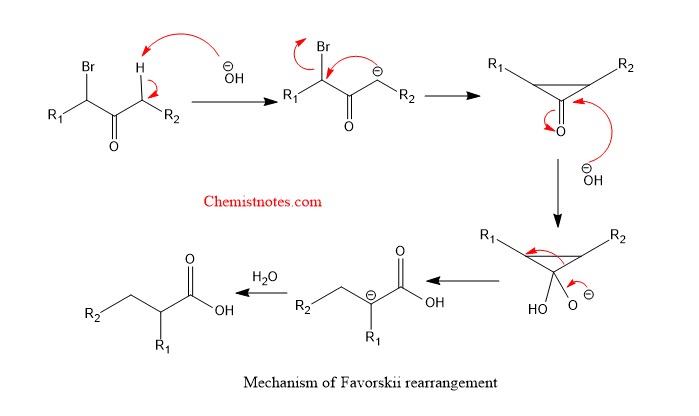
The mechanism of favorskii rearrangement is shown below:
Quasi favorskii rearrangement
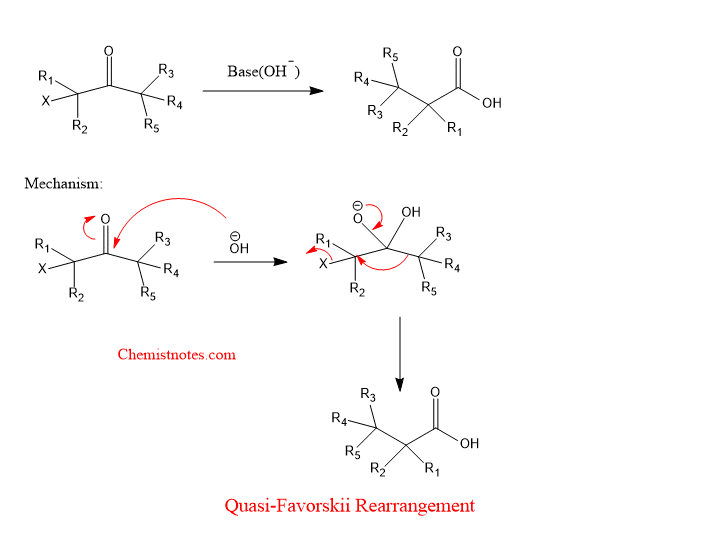
When there are no enolizable hydrogens on the alpha-halo ketones, then classical Favorski rearrangement can not take place. Therefore, a semi-benzylic mechanism takes place which is termed as abnormal Favorski rearrangement or Quasi-Favorski rearrangement.
When alkoxide or hydroxide reacts with carbonyl carbon then there occurs 1,2-migration of an alkyl group. Therefore, it is called abnormal Favorski rearrangement.
Examples of quasi favorskii rearrangement
Let’s see an example of a quasi favorskii rearrangement reaction with the mechanism.
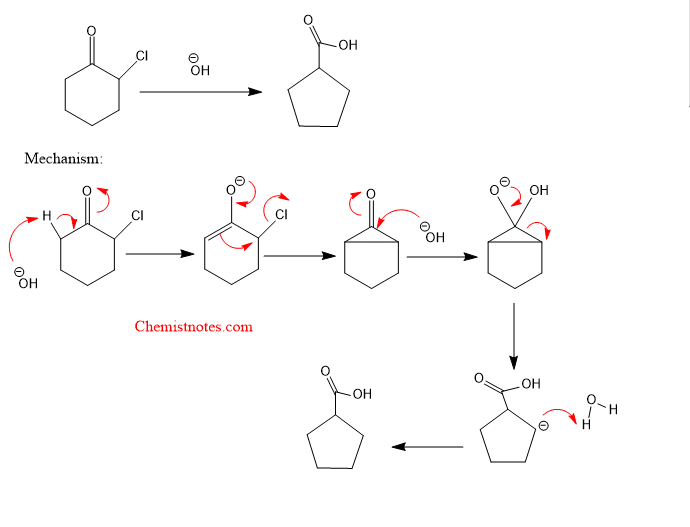
Favorskii rearrangement of cyclic ketones
Favorskii rearrangement of cyclic halo ketones results in ring contraction. Let’s see an example with the mechanism.
Favorskii rearrangement applications
Some of the applications of favorskii rearrangement reactions are:
- This reaction can be used to prepare bicyclic esters.
- Steroid and nonsteroid can be prepared by using this reaction.
- Pyrollidine can be prepared using this reaction.
Favorskii rearrangement video:
References:
- Wang, Z., Comprehensive Organic Name Reactions and Reagents, John Wiley & Sons, Inc.,2010
- J.J. Li, Name Reactions, 4th ed.,© Springer-Verlag Berlin Heidelberg 2009
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Morrison, R. T., & Boyd, R. N., Organic chemistry, Allyn and Bacon, Inc. 1987
If you want to learn about Cope Rearrangement reaction, click here.
Please comment if you have any problems related to this topic. Thank you.






