Table of Contents
ToggleBamford–Stevens reaction mechanism, examples, and applications in organic chemistry have been discussed here:
Bamford–Stevens Reaction
Bamford–Stevens reaction is such an organic reaction that leads to the formation of alkene when tosyl hydrazone of an aldehyde or ketone is treated with Na in ethylene glycol. It also employs some other strong bases such as NaOMe, NaH, LiH, NaNH2, etc. The side reactions are common and the orientation of double bond is such that highly substituted alkene is formed as a thermodynamic product. The process can be carried out in either protic or aprotic solvents.
Both the Bamford-Stevens reaction and the Shapiro reaction shares similar mechanistic pathways in forming alkenes from tosyl hydrazones.
Bamford–Stevens Reaction Examples
Some of the examples of Bamford-Stevens reactions are:

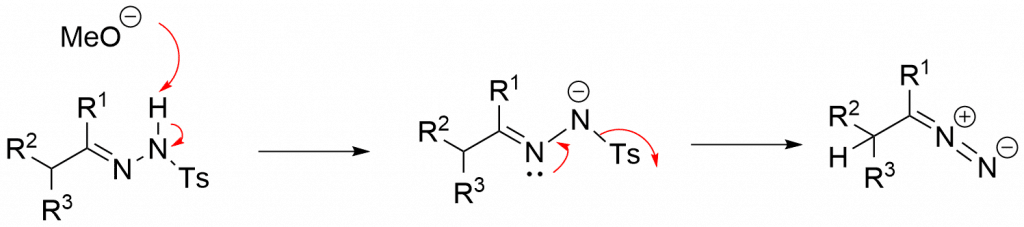
Bamford–Stevens Reaction Mechanism
There are two possible mechanisms i.e. carbenoid and a carbocation mechanism. The carbocation mechanism takes place in protic solvents, while aprotic solvents favor the carbenoid mechanism. These solvents affect the outcome of the product. Both mechanisms lead to the formation of a diazo molecule that may be isolated.
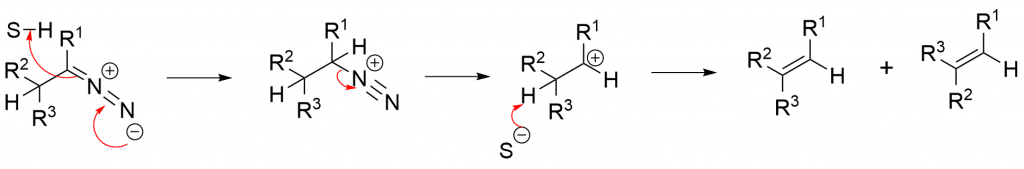
Carbocation mechanism
Here, the diazonium ion that is formed by abstracting a proton from solvent loses N2 to give corresponding carbocation, which on the loss of a proton yields a mixture of E & Z alkenes.
Carbenoid mechanism
In the presence of an aprotic solvent, the diazo compound loses N2 and yields a carbene, which by 1,2-hydrogen shift yields a Z-alkene product.
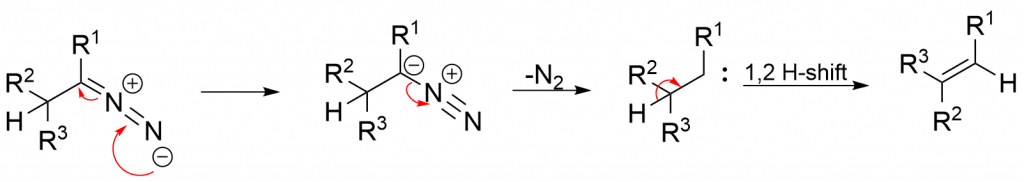
Applications of Bamford–Stevens Reaction
- This reaction is mostly used for the preparation of diazo compounds.
Bamford–Stevens Reaction Video
MCQs/FAQs
Bamford-Stevens reaction applications in pharmacy
Bamford-Stevens reaction is used for the generation of diazo compounds.
Shapiro and Bamford stevens reaction
The Bamford-Stevens reaction is a version of the Shapiro reaction. Bases such as alkyl lithium and Grignard reagents are used in the Bamford-stevens reaction, whereas bases such as Na, NaOMe, LiH, NaH, NaNH2, and others are used in the latter. As a result, the Shapiro reaction produces less-substituted olefins (kinetic products), whereas the Bamford-Stevens process produces more-substituted olefins (the thermodynamic products).
References
- March, J., Advanced Organic Chemistry, Wiley Eastern Limited, 1986.
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988.
- Paul Humphries, P. Bamford−Stevens reaction. In Name Reactions for Homologations-Part II; Li, J. J., Corey, E. J., Eds.; Wiley & Sons: Hoboken, NJ, 2009, pp642−652. (Review).
- Shapiro, R. H. Org. React. 1976, 23, 405−507. (Review).






