Table of Contents
ToggleFor a compound/molecule to exhibit electromeric effect, it must have at least one multiple bonds. Electromeric effect is a temporary effect that acts only in the presence of attacking reagent; electrophiles or nucleophiles. A compound cannot exhibit this effect if there is no attacking reagent.
Electromeric effect
The electromeric effect is a temporary effect in which dipole moment is created instantly in a molecule due to the complete transfer of a pi-electron pair by the influence of attacking reagent; electrophile or a nucleophile. In other words, an intramolecular migration of electrons from a pi bond to another atom in the molecule due to a reagent attack is defined as the electromeric effect. It is represented by E.
Example of eletromeric effect includes addition of acid to alkene, addition of nucleophile to carbonyl compounds, and so on.
Types of Electromeric effect
- +E effect (positive electromeric effect)
- -E effect (negative electromeric effect)
+E effect (positive electromeric effect)
When π-electrons of the multiple bonds are transferred towards the attacking reagent or to the atom to which the reagent is attached, π-electrons shift to a positively charged atom. Such an effect is called positive electromeric effect (+E effect). It is shown by the compound in which the attacking reagent is electrophile. Example: addition of acid (electrophile) to alkene
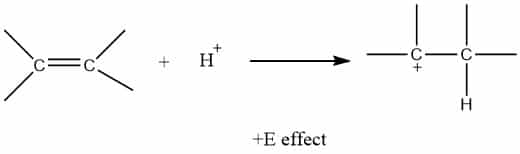
-E effect (positive electromeric effect)
When π-electrons of the multiple bonds are transferred away from the attacking reagent or transfer to the atom to which the reagent is attached, π-electrons shift to the atom away from the attacking reagent. Such an effect is called negative electromeric effect (-E effect). It is shown by the compound in which the attacking reagent is nucleophile. Example: addition of nucleophile to carbonyl compound
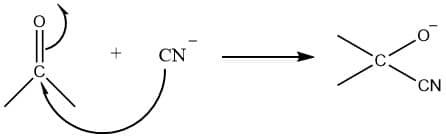
Difference between Inductive effect and Electromeric effect
| Inductive effect | Electromeric effect |
| The polarization of one bond caused by the polarization of an adjacent bond is known as an inductive effect. | Electromeric effect is a temporary effect in which dipole moment is created instantly in a molecule due to the complete transfer of a pi-electron pair by the influence of attacking reagent; electrophile or a nucleophile. |
| It is a permanent effect and irreversible. | It is a temporary effect and reversible. |
| For a molecule to exhibit inductive effect, the presence of multiple bonds is not necessary. | The molecule must have multiple bond. |
| Attacking reagents is not required. | Attacking reagents is necessary. |
| Involves displacement of sigma electrons. | Electromeric effect involves the complete transfer of pi-electrons. |
Electromeric effect and Mesomeric effect
Electromeric effect is a temporary effect in which dipole moment is created instantly in a molecule due to the complete transfer of a pi-electron pair by the influence of attacking reagent; electrophile or a nucleophile. Electromeric effect is of two types; +E effect and the -E effect. +E effect. +E effect occurs when π-electrons of the bond are transferred to the attacking reagent, while -E effect occurs when π-electrons of the bond are transferred to the atom to which the attacking reagent is not attached.
Mesomeric effect is defined as the polarity produced in a molecule or a conjugated system by the movement of π electrons towards or away from a substituent group. Mesomeric effect is of two types; +M effect and the -M effect. +M effect occurs when the π electrons move away from the particular group towards the rest of the molecule, and increases the electron density of the molecule/conjugated system. -M effect occurs when the π electrons are moved from the rest of the molecule to a particular group, the electron density of the molecule/conjugated system decreases.
Difference beween electromeric and Mesomeric effect
| Electromeric effect | Mesomeric effect |
| Electromeric effect is a temporary effect in which dipole moment is created instantly in a molecule due to the complete transfer | Mesomeric effect is defined as the polarity produced in a molecule or a conjugated system by the movement of π electrons towards or away from a substituent group. |
| Electromeric effect is of two types; +E effect and the -E effect. | Mesomeric effect is of two types; +M effect and the -M effect. |
| It is due to the substituents, either electron-withdrawing or electron-donating in a conjugated system. | It occurs due to the presence of attacking reagents; either electrophile or nucleophile. |
Electromeric effect Video
FAQs
What is electromeric effect?
Electromeric effect is a temporary effect in which dipole moment is created instantly in a molecule due to the complete transfer of a pi-electron pair by the influence of attacking reagent; electrophile or a nucleophile.
explain the term inductive and electromeric effect
The polarization of one bond caused by the polarization of an adjacent bond is known as an inductive effect.
An intramolecular migration of electrons from a pi bond to another atom in the molecule due to a reagent attack is defined as the electromeric effect.
electromeric effect in carbonyl compound
The addition of nucleophile to carbonyl compound exhibits negative electromeric effect (-E effect).






