Table of Contents
ToggleTransition state theory or activated complex theory is also known as absolute reaction rate theory because with its help it is possible to get the absolute value of the rate constant. Transition state theory was first given by Marcellin in 1915 and later developed by Polanyi and Erying in 1935.
Transition state theory
According to transition state theory, the reactant molecules are first transformed into an intermediate transition state or activated complex. Then the activated complex is unstable and breaks into the product at a certain rate.
Reactants ⇌ Activated complex → Product
The transition state theory may be summarised as:
1. There exist equilibrium between activated complex and reactant molecules. Once the transition state is formed it can either return to the initial reactants or proceed to form the products.
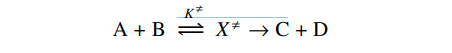
The concentration of activated complex can be obtained by applying the equilibrium as,
[X≠] = K≠[A][B]…………………………………..(i)
where ≠ refers to the activated complex.
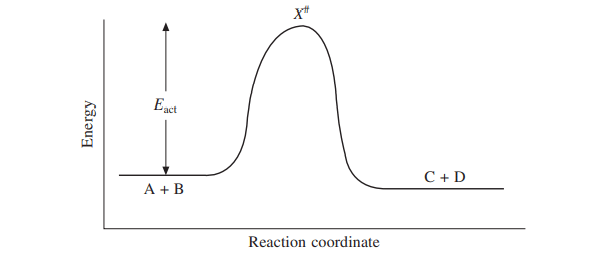
2. Energy of activation is the additional energy that must be supplied to the reactants in order to form an activated complex as shown below.
3. The vibrational degree of freedom of the activated complex is quite unstable and is responsible for the dissociation of the activated complex to form the product. The frequency of such vibration will be low and average energy will be of the order of kbT.
i.e. Evib = kbT = hν
or, ν = kbT/h ………………………(ii)
where, kb= Boltzmann’s constant
h= planck’s constant.
4. The rate at which the complex breaks up into the products depends on the concentration of the activated complex and the frequency of vibration of the activated complex.
Rate = ν [X≠] …………………………………………….(iii)
Putting the values of [X≠] and ν in equation (iii) from (i) and (ii)
Rate=(kbT/h ) ⨉ K≠[A][B] …………………………….(iv)
If k is the rate constant for the above reaction then,
A + B → Products
The experimental rate of reaction=k[A][B] ……………………(v)
From equations (iv) and (v), we get,
k= (kbT/h ) ⨉ K≠ …………………………..(vi)
This is the required transition state theory equation.
Advantages of transition state theory
The advantages of transition state theory are:
- The value of activation energry and pre-exponential factor A can be calculated.
- The value of steric factor can also be predicted.
- This theory is applicable to both gaseous and aqueous reaction.
- It provides the complete discription of nature of reaction including the changes in structure and distributin of energy through the activated complex.
Transition state theory vs collision theory
The difference between collision theory and transition state theory is given as:
| Transition state theory | Collision theory |
| 1. Transition state theory states that reaction occurs via the formation of the transition state | 1. Collision theory states that reaction occurs via collision of reactant molecules with sufficient energy and proper orientation |
| 2. It is applicable to both gaseous and aqueous reactions. | 2. It is applicable to gaseous reactions. |
| 3. Transition state theory explains the cleavage and formation of bonds in the transition state. | 3. Collision theory does not explain the cleavage and formation of bonds in the reaction. |
Transition State theory video
For more details:






