Table of Contents
ToggleBenzoin condensation was first of all reported by Stagne in 1824, which was catalyzed by cyanide ions. Cyanide catalyzed condensation of aryl aldehyde to benzoin is called benzoin condensation.
Benzoin condensation reaction
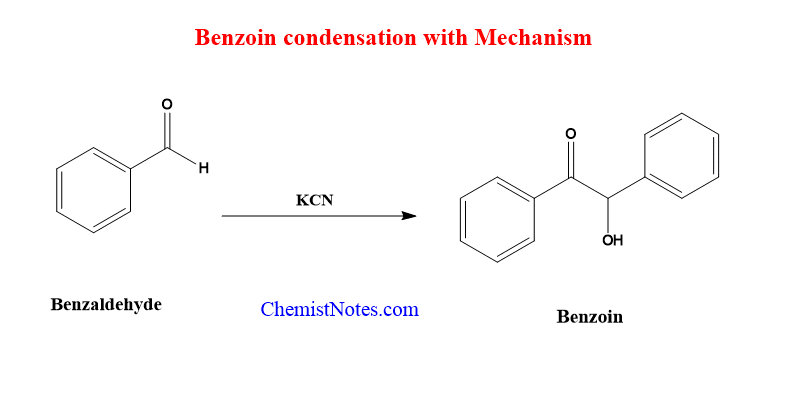
When aromatic aldehydes are treated with cyanide anions, they undergo a condensation reaction that results in the formation of alpha hydroxyl ketones, also known as benzoins (KCN). This is known as Benzoin condensation.
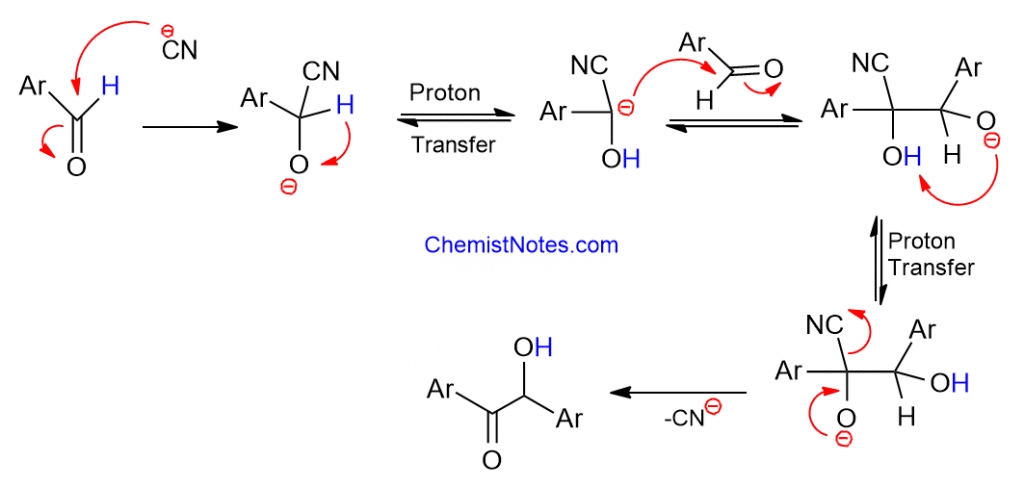
The cyanide ions are significant in this reaction because, in addition to being a good nucleophile, their electron-withdrawing effect allows for the production of carbanion species via proton transfer, and they are a good leaving group. Because of these properties, the cyanide ion acts as a particular catalyst for benzoin condensation.
Nowadays, cyanide is replaced by a number of reagents. The original cyanide-catalyzed benzoin condensation has been modified to employ thiamin and a similar thiazolium salt, diamine, or enzyme as a catalyst.
Benzoin condensation reaction with mechanism
As a nucleophile, the cyanide ion adds to the aldehyde molecules, resulting in the anionic species. The neighboring cyano group increases the acidity of the aldehydic proton, allowing the formation of tautomeric carbanion species and the addition of another aldehyde molecule. In the following steps, the product molecules are stabilized by the loss of the cyanide ion, yielding benzoin.
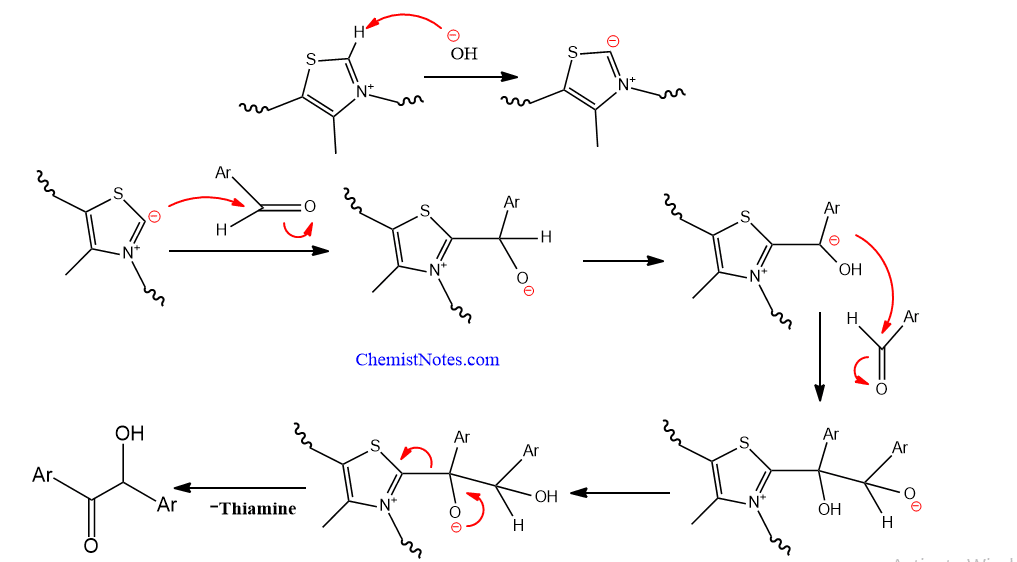
Benzoin condensation with thiamine
In recent years, benzoin condensation is carried out in presence of thiamine instead of cyanide ion. The final product is benzoin but the mechanism is slightly different.
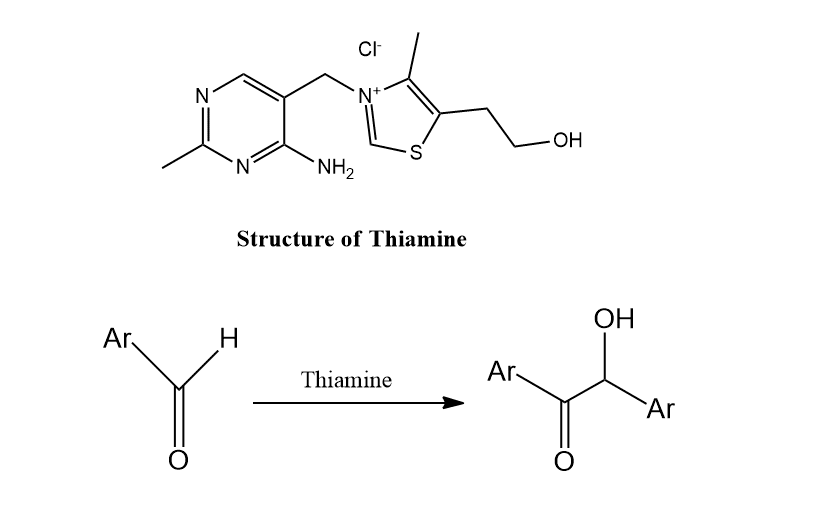
Benzoin condensation with thiamine mechanism
The mechanism of this reaction involves the nucleophilic attack of thiamine on the carbonyl carbon of benzaldehyde resulting in the formation of benzoin as shown below:
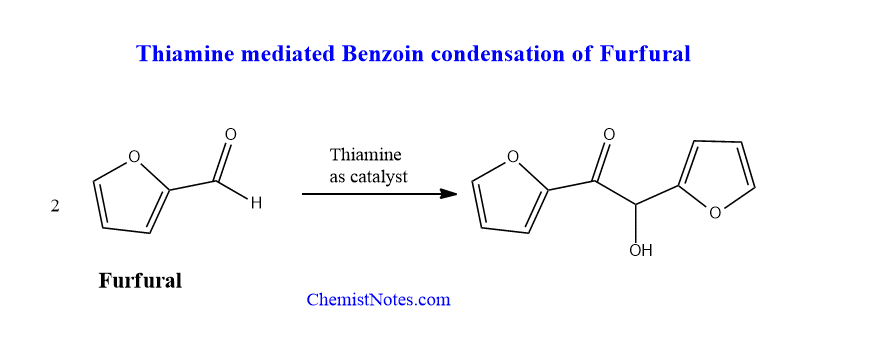
Thiamine mediated benzoin condensation of furfural
Furfural can undergo benzoin condensation in presence of thiamine or cyanide ion as the catalyst. The mechanism is the same as the above.
Cross benzoin condensation
When two different aldehydes undergo benzoin condensation in presence of cyanide ion to give a mixture of products in different ratios. This reaction is known as cross benzoin condensation.
Benzoin condensation importance
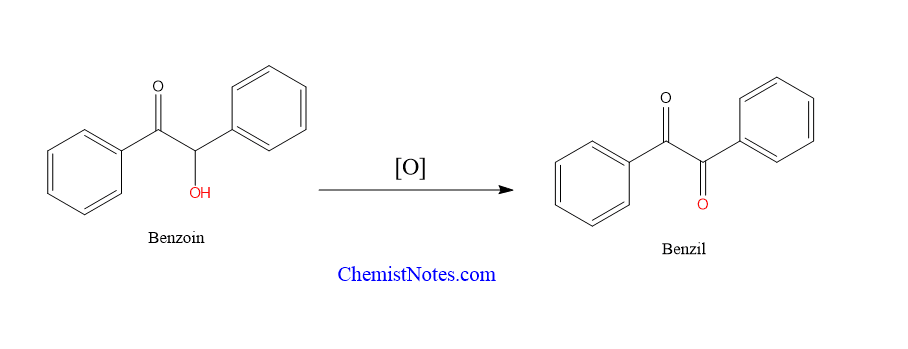
This reaction produces benzoins, which can be oxidized to benzyl or transformed into various diphenyl alpha-amino ketones or alcohols. This is one of the major applications of benzoin condensation.