Table of Contents
ToggleChichibabin amination reaction was first of all reported by Chichibabin in 1914. This reaction involves the formation of the amino derivative compound of pyridine. The general reaction, mechanism, example, and application of this reaction have been discussed below:
Chichibabin amination reaction
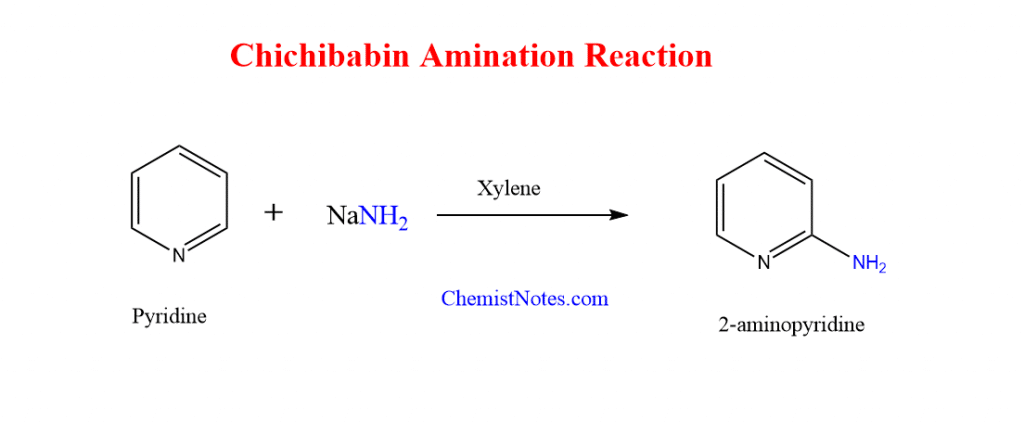
Pyridine forms 2-aminopyridine when it reacts with sodium amide in boiling inert solvents like toluene, xylene, or benzene. This reaction is known as chichibabin amination or chichibabin reaction. In this synthesis, sodium amide or potassium amide can be used as an amine source.
One remarkable aspect of this reaction is that the yield of 2-aminopyridine is very dependent on the condition of the sodium amide utilized in the synthesis. For example, the purest sodium amide does not react much with pyridines, but less pure sodium amide yields well. It could be because of the catalytic impact of the impurities in the sodium amide.
As the amount of sodium amide increases, a second amino group may be inserted into the aromatic ring. But, When substituents block both positions 2 and 6, amine groups will be inserted into position 4 with a low yield.
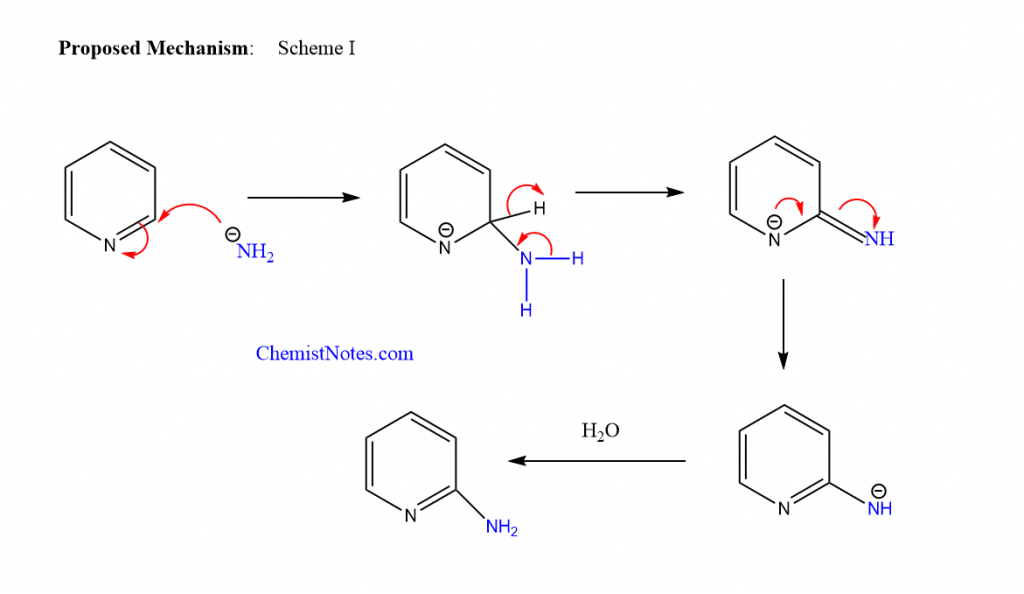
Chichibabin amination mechanism
There are two proposed mechanisms for this reaction. The reaction may involve the attachment of an amide anion to the pyridine ring may be followed by hydrogen elimination and the synthesis of aminopyridine upon hydrolysis.
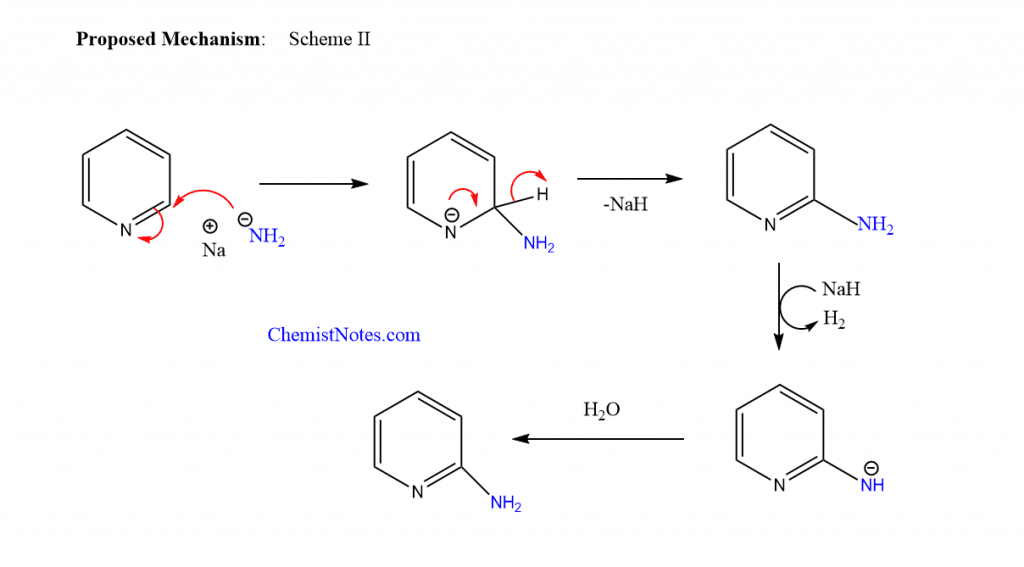
Similarly, another proposed mechanism involves the addition of an amide anion to the pyridine ring, followed by the elimination of hydride, which then deprotonates at the amino group.
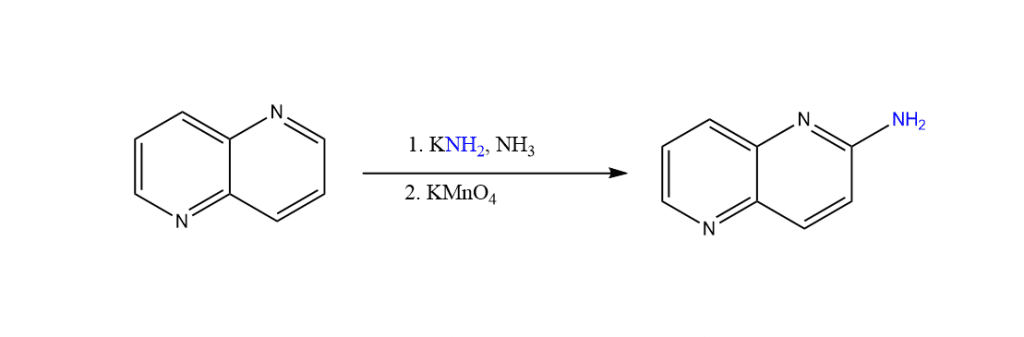
Chichibabin amination application
This reaction is useful in the synthesis of aromatic rings containing nitrogen.. In the traditional process, nitration was followed by reduction, which is no longer relevant or works badly.






