Table of Contents
ToggleAmbident nucleophile is a type of nucleophile which have two attacking sites, and thus has chances of producing a mixture of products. More details of it, along with definition, examples, and regiochemistry have been discussed in this post.
Ambident nucleophile
We are familiar with the term nucleophile, aren’t we? Actually, the nucleophile is electron-rich species, which may be either negatively charged (OH–) or neutral species having unshared pair of electrons. Nucleophiles thus attack the electron-deficient center i.e positive center, to form bonds. Simple nucleophiles generally have one atom which bears either a negative charge or unshared pair of electrons, hence such nucleophiles are capable of attack by that negatively charged atom to give a product.
But there are some nucleophiles that contain unshared pairs of electrons in more than one atom, which are termed Ambident nucleophiles.
Ambident nucleophile definition
Some nucleophiles have a pair of electrons on each of two or more atoms. In other words, the canonical forms of nucleophiles can be drawn in which two or more atoms bear an unshared pair. Therefore, the nucleophiles may attack in two or more different ways to give different products. Such nucleophiles are called ambident nucleophiles.
Ambident nucleophile examples
Common examples of ambident nucleophiles are cyanide ion (CN–), and nitrites ion(NO2-). There are other ions formed by the abstraction of the proton, which also acts as nucleophiles.
- CN– ions: it can give either RCN(Nitriles) or RNC(Isocynides). It has two atoms C and N-atom by which it can attack the substrate. If it attacks by its C-atom, it gives RCN and if it attacks by N-atom, it forms RNC.
- NO2– ion: It can give either nitrile ester (R-O-N=O) or nitro (RNO2). Nitrile ester is formed when NO2– ion attacks by its O-atom and nitro is formed when it attacks by its N-atom.
- Other ions: These are derived by the removal of a proton from malonic ester, Beta-ketoester, Beta-diketones, etc. Some of these ions are shown below in the figure.
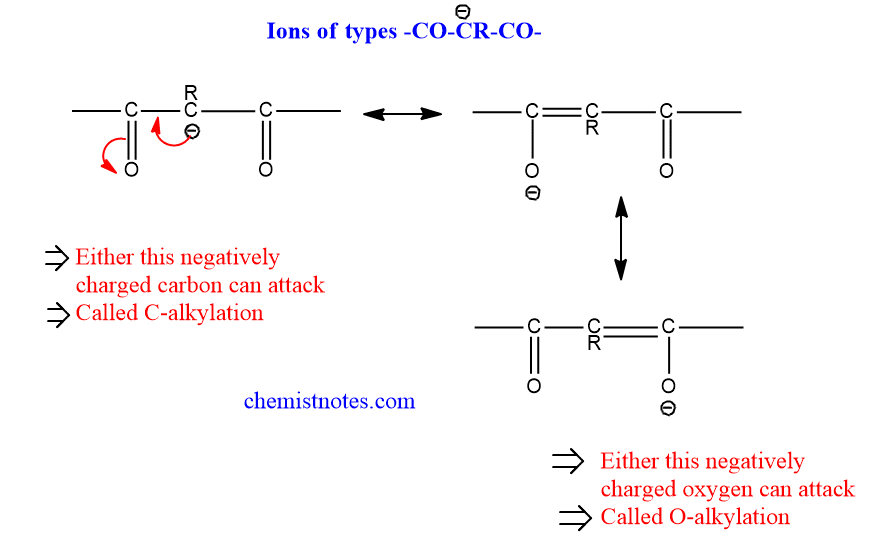
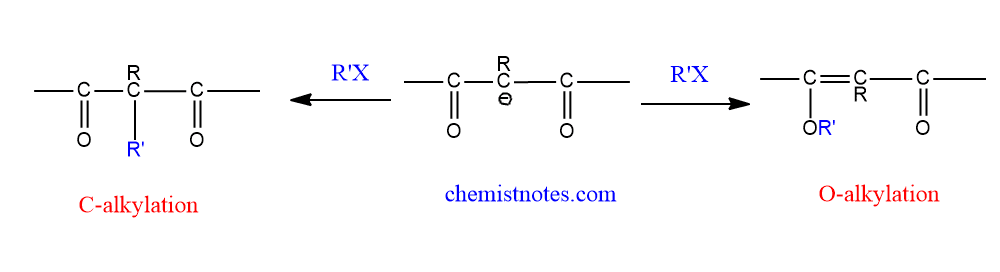
These ions can attack a saturated carbon with either carbon atoms, which is called C-alkylation, or with their oxygen atom, which is called O-alkylation.
Ambident nucleophile and regioselectivity
Most nucleophiles with two potentially attacking atoms can attack with any of them depending on circumstances, and mixtures of two products are frequently formed, however, this is not always the case. A reaction is considered to be regioselective when it has the capacity to create two or more structural isomers but only produces one.
The regioselectivity in the case of ambident nucleophiles can be explained on the basis of the concept of the hard-soft acid-base (HSAB Principle). One important fact must be considered. “More electronegative atom of an ambident nucleophile is a harder base than a less electronegative atom.”
If the reaction condition is made to proceed through the SN1 mechanism, the nucleophile must attack carbocation, which is harder acid. In such a case, carbocation ( harder acid, don’t forget) is only attacked by the more electronegative atom (harder base) of the ambident nucleophile and thus only one product is formed.
Similarly, In the SN2 mechanism, the nucleophile must attack the carbon atom, which is softer acid. In such a case, the carbon atom(softer acid) is only attacked by the less electronegative atom( softer base) of the ambident nucleophile, and only one product is practically formed.
Therefore, when the character of a given reaction is changed from SN1 to SN2-like, an ambident nucleophile becomes more likely to attack with its less electronegative atom.
This can be illustrated by an example. When conditions of the reaction are changed from SN1 to SN2 condition, C-attack by CN– is favored. Similarly, changing such conditions from SN1 to SN2, N-attack by NO2– is favored instead of O- attack.
Ambident nucleophiles youtube video
References
- J. March, Advanced Organic Chemistry, (4th Edition), John Wiley and Sons, 1992