Table of Contents
ToggleThe separation of lanthanides elements is difficult since their chemical properties are almost identical. The properties of metal ions are mainly determined by their size and charge. The lanthanides are all typically trivalent (charge) and are almost similar in size, and hence possess difficulty in separation. Only two methods are currently used for separating the lanthanides:
- Ion Exchange Method
- Valency Change Method
Separation of Lanthanides by Ion Exchange Method
For the separation and purification of Lanthanides, the ion-exchange method is the most significant, rapid, and reliable technique.
A lanthanide ion solution is passed through a column of -SO3H containing synthetic ion-exchange resin (such as Dowex-50). The Ln3+ ions bind to the resin by replacing H+ ions in the SO3H groups.

Thus, the Ln3+ ions get fixed to the resin, and the H+ ions produced are washed through the column.
Then, a buffered solution of citric acid/ammonium citrate (eluent) is passed through the resin. The Ln3+ ions are eluted/removed by this solution, which forms stable complexes with the citrate ion and moves down slowly. The smaller Ln3+ ions form citrate complexes first and possess more stability as compared to other citrate complexes. As a result, the lanthanide with smaller ions discharges out of the column first.
Since the ions leave the column at different speeds, separation is feasible. The solution coming out of the column is collected in various containers in various fractions; each container receives a solution component (elute) that is rich in a certain metal. The metal ion is precipitated as its oxalate when ammonium oxalate solution is added to each solution component, which when heated forms the oxide.
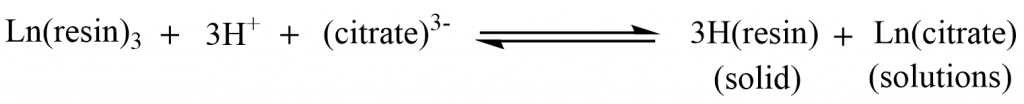
Separation of Lanthanides by Valency Change Method
The ability of some of the lanthanides to exist in variable valencies is employed in separating these elements.
Ce4+ is a stable ion that differs from Ce3+ and other Ln3+ ions in some properties. This property is used to distinguish Ce from other lanthanides. When NaOCl is added to a mixture of Ln3+ ions, Ce3+ is oxidized to Ce4+ resulting in the precipitation of The Ce4+ as Ce(OH)4 from the mixture, which can then be heated to generate CeO2. Alternatively, tributyl phosphate can be used to extract Ce4+ ions.
When compared to other Ln3+ ions, Eu2+ has quite unique properties. The Eu3+ ions in the mixture are reduced electrostatically to Eu2+ and then precipitated as EuSO4 before being filtered out of the solution with the other (unaffected) Ln3+ ions. The separation of Sm and Yb via their divalent species follows a similar procedure.
The other two methods; the separation of lanthanides by fractional crystallization method and the liquid-liquid extraction method can be also applied for the separation of lanthanide elements.
- Fractional Crystallisation: This approach is based on the formation of isomorphous compounds with considerable variances in solubilities, as well as significant changes in solubilities as a function of temperature. The double nitrates of Mg and Ce, for example, can be distinguished from the double nitrates of Mg and other lanthanides.
- Liquid-liquid Extraction Method: In organic solvents, the solubilities of Ln3+ ions differ greatly. The metal in the combination may be complexed first, followed by the transfer of one or more of the products to the organic solvent layer. This approach can be used to extract Ce(IV) and Th(IV) from other Ln3+ ions. They produce H2 when they react with dilute mineral acids.
Separation of Lanthanide elements Video
FAQ/MCQs
What are the methods employed for separation of lanthanides?
Ion exchange method and Valency change method are two common methods employed for the separation of lanthanides.
Why separation of lanthanides is difficult?
The separation of lanthanides is difficult due to identical chemical properties; +III charge trivalent (charge) and similar in size.
References
- J. D. Lee, Concise Inorganic Chemistry, 5th Edition, John Wiley and Sons. Inc. 2007.
- F. A. Cotton, G. Wilkinson & C. Gaus, Basic Inorganic Chemistry, 3 rd Edition, John Wiley & Sons (Asia), Pvt., Ltd., 2007.
- D. F. Shriver & P. W. Atkins, Inorganic Chemistry, 5th Edition, Oxford University Press, 2010.






