Table of Contents
ToggleThe Half life formula, a measure of the radioactive element gives information about the time required for the disintegration of one-half of the radioactive nuclei presents initially in the sample. Half life or half life period of a radioactive element is defined as the time required for the disintegration of one-half of the original amount of radioactive element. It is denoted by t1/2.
Half-life is a characteristic unit for the exponential decay equation and is constant across a quantity’s lifetime. The shorter the t1/2, the greater the number of atoms decaying in unit time. It may vary from a fraction of a second to millions of years.
How to calculate half life?

If t1/2 is the half-life period of a radioactive element, then at t1/2
N = 1/2 No ………………(i)
Substituting this value into rate equation for radioactive disintegration (v), we get
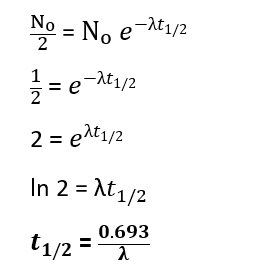
This is the required half life equation for a radioactive element.
Half life formula
As per the above-derived equation, the half-life formula for the radioactive element is
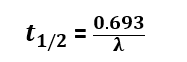
Relation between t and t1/2
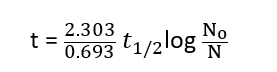
Significance of half-life period
An element’s half-life time provides information on its stability. In comparison to isotopes with shorter half-lives, those with longer half-lives are generally more stable. Some of the major significance of the half-life period are:
- The half life period is independent of the initial amount of radioactive substance,
- A radioactive substance’s concentration is decreased by half after each half-life, and as a result, its activity is lowered by half as well. It may be calculated that the activity reduces to (1/2)n when ‘n’ half-life periods have passed. The following relation gives the amount of substance remaining after ‘n’ half-life periods.
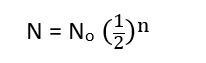
Half life video
FAQs
half life of uranium
The half life of uranium 238 is about 4.5 ×109 years, while that of uranium 235 is 700 million years.
half life symbol
half life symbol is t1/2
What is the half life of carbon 14?
the half life of carbon 14 is about 5700 ± 30 years.
half life of uranium 238
The half life of uranium 238 is about 4.5 ×109 years.
half life of uranium 235
The half life of uranium 238 is about 700 million years.
References
- Atkins, P. (2010). Shriver & Atkins’ Inorganic Chemistry (5th or later Edition). Oxford University Press.
- Lee, J. D. (2008). Concise Inorganic Chemistry: Fifth Edition by J.D. Lee (Fifth edition). Oxford University Press.
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.






