Table of Contents
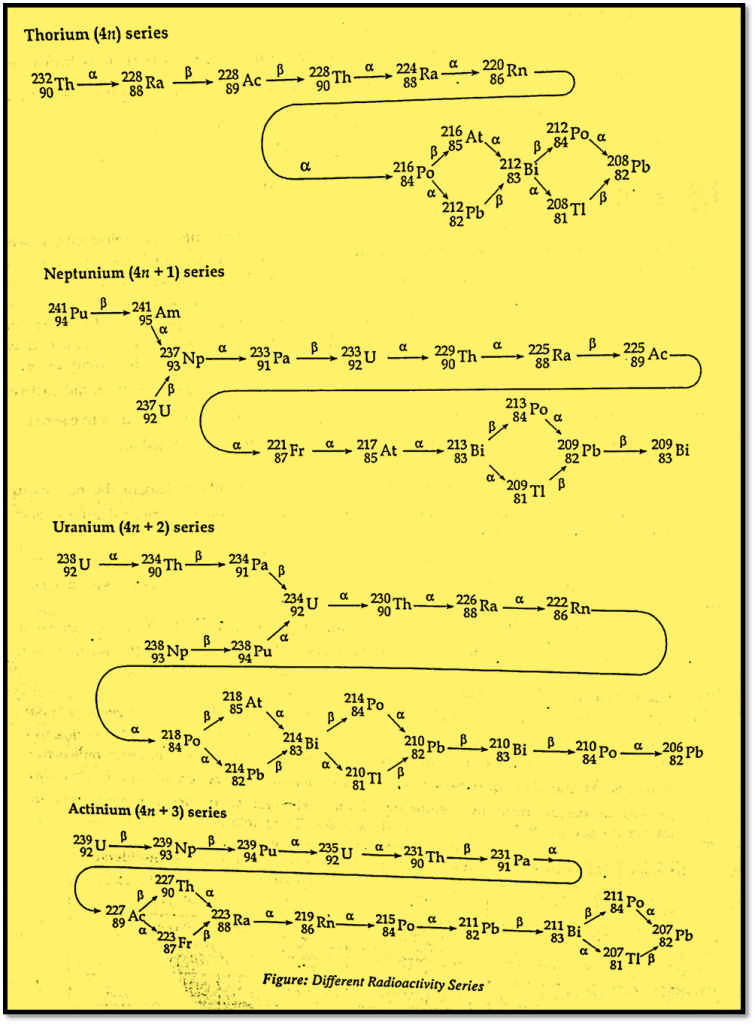
ToggleThe radioactive series also called as radioactive decay series is the series of elements obtained by successive disintegration of a parent radioactive element to a non-radioactive stable element. The series of elements obtained from 90Th-232, 92U-235, and 92U-239 are natural radioactive series, while the series obtained from artificially prepared 93Np-237 is called radioactive series.
When a radioactive element emits α or β particles, the new daughter elements are formed and may have unstable nuclei. The daughter element further disintegrates by emitting α and β particles forming new elements. The process of disintegration is continued until a non-radioactive stable element is obtained. Thus, the formation of the stable nucleus from a radioactive element is not a one-step process.
There are four series of radioactive elements.
- Thorium (4n) Series
- Uranium (4n+2) Series
- Actinium (4n+3) Series
- Neptunium (4n+1) Series
Thorium (4n) Series
It begins with the radioactive elements, Uranium-232 and ends with stable non-radioactive lead (Pb)-208. The mass number of all elements are integral multiple of 4.
Uranium (4n+2) Series
The parent element, uranium-238, is used as its starting parent radioactive elements which undergo disintegration and form lead-206 as its final stable component. Since the mass number of all elements in this series gives a remainder of 2 when divided by 4, it is called the (4n+2) series, where n is an integer. It possesses the longest half-life.
Actinium (4n+3) Series
The radioactive element uranium-235 converts into Lead-207, a stable element as the final product. Since the mass number of all elements in this series gives a remainder of 3 when divided by 4, it is called the (4n+3) series, where n is an integer.
Neptunium (4n+1) Series
The elements in this series are not found in nature. It begins with Bismuth-200 and terminates into stable neptunium-237. The mass number of all elements in this series gives a remainder of 1 when divided by 4, hence is called the (4n+1) series.
List of radioactive series
The various radioactive series with their starting element, end-stable elements, and the half-life is presented below:
| Series | Name of Series | Starting Radioactive Element | Stable Non-Radioactive Element | Half-life of Starting Element |
| 4n | Thorium | 90Th-232 | 82Pb-208 | 1.3 ×1010 year |
| 4n+1 | Neptunium | 94Pu-241 | 83Bi-209 | 4.5 ×109 year |
| 4n+2 | Uranium | 92U-238 | 82Pb-206 | 7.1 ×108 year |
| 4n+3 | Actinium | 92U-239 | 82Pb-207 | 13.2 year |
Radioactive Series Video
References
- Atkins, P. (2010). Shriver & Atkins’ Inorganic Chemistry (5th or later Edition). Oxford University Press.
- Lee, J. D. (2008). Concise Inorganic Chemistry: Fifth Edition by J.D. Lee (Fifth edition). Oxford University Press.
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.






