Table of Contents
Toggled block elements are those elements in which the last electron (differentiating electron) enters (n-1) d orbitals i.e d-orbitals of the penultimate shell. This block corresponds to groups 3 to 12 in the periodic table. d block elements are often also called transition metals but according to IUPAC, transition metals are those elements that have an incomplete d subshell or elements that can form a cation with an incomplete d-subshell. Thus, group 12 metals Zinc, Cadmium, and Mercury have not typically considered transition metals.
d block elements
The elements in groups 3 to 12 in the periodic table are defined as d-block elements. The general valence shell electronic configuration of d-block elements is (n-1)d 1-10 ns 1-2
Classification of d block elements
On the basis of electronic configuration and the last electron which enters on 3d, 4d, 5d, and 6d orbitals, d-block elements are further can be classified as:
- 3d series: In atoms of these elements, the last electrons enter the 3d orbitals. These series have a total of 10 elements ranging from Scandium(Z=21) to Zinc(Z=30).
- 4d series: In atoms of these elements, the last electrons enter the 4d orbital. These series have 10 elements ranging from Y39 to Cd 48.
- 5d series: In atoms of these elements, the last electrons enter the 5d orbital. This series has 10 elements. One is La57 and the other nine elements range from Hf72 to Hg80. The other 14 elements( from Ce58 to Lu71) which are present in between La to Hf are known as Lanthanides or lanthanone.
- 6d series: In atoms of these elements, the last electrons enter the 6d orbital. These series have 4 elements which are Ac89, Ku104, Ha105, and Unh106. Therefore, this series is incomplete series. There are 14 elements from Th90 to Lw103 in between Ac89 to Ku104, known as Actinides.
List of d block elements
All d block elements names are listed below:
- 3d series: Scandium(21), Titanium(22), Vanadium(23), Chromium(24), Manganese(25), Iron(26), Cobalt(27), Nickel(28), Copper(29) and Zinc(30).
- 4d series: Yttrium(39), Zirconium(40), Niobium(41), Molybdenum(42), Technetium(43), Ruthenium(44), Rhodium(45), Palladium(46), Silver(47), Cadmium(48).
- 5d series: Hafnium(72), Tantalum(73),Tungsten(74), Rhenium(75),Osmium(76), Iridium(77), Platinum(78), Gold(79) and Mercury(80).
- 6d series: Rutherfordium(104), Dubnium(105), Seaborgium(106), Bohrium(107), Hassium(108), Meitnerium(109), Darmstadtium(110), Roentagenium(111) and Copernicium(112).
Properties of d block elements
Atomic radii of d block elements
Let’s discuss the variation of atomic radii in the period. Actually, the atomic radii of elements of a particular period decrease gradually up to the middle elements and then remain constant. But, the last element shows an increase in its atomic radius.
For example: In the 3d series, atomic radii decrease gradually from Sc to Mn but the value remains practically constant from Fe to Cu. And, the atomic radius of Zn increases. This trend can be explained on the basis of effective nuclear charges.
From Sc to Mn, the atomic radius decreases because of the gradual increase in nuclear charge with the increase in atomic number. The increase in effective nuclear charges causes decreases in the size of an atom.
Due to the screening effect caused by 3d electrons, the magnitude of nuclear charges decreases, and hence the elements from Fe to Cu the value of atomic radii remains the same. In the case of Zn, the electron-electron repulsion increases and becomes greater than nuclear attraction thus the size of Zn increases more than Cu.
Along the column, from top to bottom, the size of atomic radii increases with increases in the atomic numbers.
Ionization Potential of d block elements
d-block elements have higher ionization energy than s-block elements and lower than those of p-block elements. In the 3d series, the values of ionization energy increase as we move from left and right in the series i.e Sc to Zn, although the increment is not quite regular i.e the increase in ionization energies is quite gradual or slow.
The main reason behind such a trend is that when we move from left to right i.e Sc to Zn, the nuclear charge increases. The increased nuclear charge would attract the outer electron cloud with greater force. Hence, ionization energy is expected to increase in each step.
However, as the electron is added to the 3d subshell at each next element, outer electrons are shielded more and more. The effect of increasing nuclear charges is opposed by the additional screening effect of the nucleus and consequently, the ionization energy increase but quite slowly.
In the series, Zn has the highest value of ionization energy which is due to the extra stability associated with their completely filled 3d and 4s orbitals.
Variable oxidation state of d block elements
Most d block elements show variable oxidation states i.e more than one oxidation state. The main reason behind the properties of such elements is given below.
- Due to similar energy (almost the same) of (n-1)d and ns orbitals of d-block elements, electrons can be easily removed from (n-1) d orbitals similar to ns orbitals.
- When all ns electrons are removed, only the penultimate shell remains. In this case, the penultimate shell becomes unstable and can lose one or more electrons to gain stability which results in the formation of cations having different oxidation states.
Some of the elements showing variable oxidation states are given below.
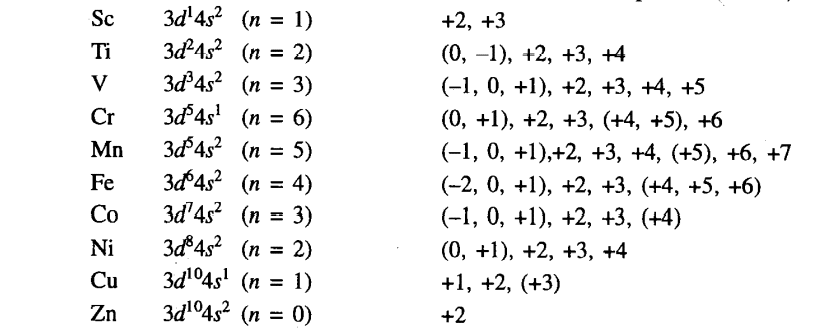
Complex formation by d block elements
Transition metals or their cations have been reported to form a large number of complex compounds with many neutral molecules such as CO, NO, or NH3 or ions such as F–, Cl–, CN– etc, these are known as ligands.
These ligands have one or more lone pairs of electrons which can be easily donated to the transition metal atom or cation forming a Ligand-metal coordinate bond. For example, Fe[(CN)6]3-
The main reason for the complex formation tendency of these metals are:
- d-block elements’ atoms or cations usually have vacant d-orbitals thus the lone pair donated by ligand can be easily accumulated in these vacant orbitals. Hence, a Ligand-metal bond is formed.
- These metals cations have small size and have high effective nuclear charges thus they can easily attract the lone pair of electrons from the ligands.
Colour compounds of d block elements
Transition elements usually form colored compounds. The splitting of d-orbitals of metal ions takes place under the influence of the surrounding ligands. The d-orbitals are split by the surrounding ligands into two sets of orbitals of low and high energy.
The energy gap between these two levels is comparatively small, consequently, the excitation of an electron from the lower to higher d-levels can be achieved by absorption of visible light. This causes the transition metal compounds to appear colored.
They usually form colored compounds. This is due to incomplete filling of d-orbitals where absorption of light could lead to the d-d electronic transition from the visible region. Zn2+, Cu+, etc. don’t usually exhibit colored compounds due to the complete filling of d-orbitals and hence d-d transition is not feasible.
Similarly, TiO2 is white because Ti4+ has zero electrons in d-orbitals i.e d0. In the series, Sc3+, Ti4+, V5+, etc. ions may be considered to have empty d-shell, hence d-d spectrum are impossible and should be colorless. Thus, transition metal compounds having central metal ions with empty d-orbitals(d0) or completely filled d-orbitals ( d10 case) are colorless.
The colors of the absorbed light and transmitted light are different from each other. The color of transmitted light is called the complementary color of that absorbed light and the color of the transmitted light is the color of the compounds in our eyes.
For example, Cu2+ Salts absorb yellow radiation, it transmits blue radiation which is the complementary color of yellow light, and hence this salt looks blue to our eyes.`
Magnetic behavior of d block elements
When a substance is placed in the magnetic field of strength H, the intensity of the magnetic field induced by the substance Hc may be greater than or less than H. If the field in the substance is greater than H, the substance is paramagnetic.
A paramagnetic substance is one which attracted to the magnetic field. If the field in the substance is less than H, the substance is diamagnetic. A diamagnetic substance is one that is repelled by a magnetic field.
Paramagnetism arises from the presence of unpaired electrons in atoms, ions, complexes, or molecules. However, the magnetic momentum(u) of transition elements and their compound is related to the number of unpaired electrons.
The paramagnetism first increases in any transition series elements to the middle and decreases up to the end. So, the paramagnetic character of 3d-series elements increases from Sc to Mn and then decreases up to Zn.
Catalytic properties of d block elements
Most of the transition metals and their compounds exhibit catalytic properties either in organic or inorganic reactions. The most used elements are Fe, Pt, Pd, and Ni, and compounds including V2O5.
In some cases, the transition elements provide unpaired d-electrons to form unstable intermediate compounds with reactants, while in other cases, transition metals provide a large surface area for the reactant to be adsorbed.
The ability to exhibit the catalytic properties due to mainly following properties of transition metals.
Formation of intermediate compound
Almost all catalytic reaction proceeds through the formation of an intermediate between the reactant and catalyst. So, in order to exhibit the catalytic properties, the substance should form an intermediate. In order to form the intermediate, the transition state element should change its original oxidation.
Since transition metals exhibit a variable oxidation state, they can form a variety of compounds’ intermediates by using a suitable oxidation state. Chemist believes that the catalytic activity of the transition metals depends on their ability to exist in a different state of oxidation or coordination.
Formation of interstitial compounds
Transition metals are also capable to absorb reacting species and forms interstitial compounds. By doing so, the reacting species are activated, and hence the rate of reaction increases.
Adsorption
Transition metals have the unique ability to adsorb reactant molecules on their surface. Transition metal provides a large surface area to the reactant for reaction. Adsorption of reactant molecules on the surface of the catalyst weakens the bonds in the reactant and facilities the reaction.
Examples:
- Ni metals act as catalysts in hydrogenation reactions.
- Fe acts as a catalyst in Haber’s process.
- V2O5 acts as a catalyst in the contact process for the manufacture of H2SO4.
- TiCl4 acts as a catalyst in the polymerization of ethene into polyethylene.
d block elements video
.
FAQs/MCQs:
What are d block elements?
d block elements are those elements whose last electron enters the d-orbital.






