Table of Contents
ToggleWhat are isoelectronic species?
Species such as atoms, molecules, or ions having the same number of electrons are called isoelectronic species. This term is made up of two Greek words Iso+electronic, “Iso” means the same, and electronic is related to electrons.
Properties of isoelectronic species
Some general properties of isoelectronic species have been discussed below:
- These species have different atomic numbers, and atomic masses (sum of protons and neutrons) but the number of electrons is the same.
- The atomic radius of isoelectronic species is also different.
- Isoelectronic species have the same electronic configuration.
- Due to the same electronic configuration, they may have the same electronegativity value, ionization energy value as well as chemical reactivity.
Examples of isoelectronic species
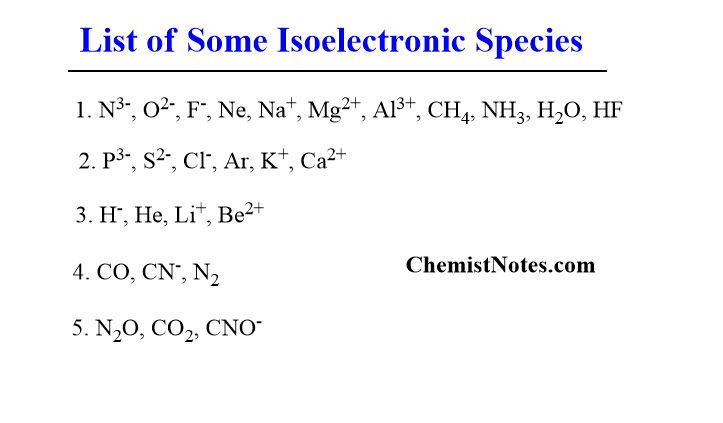
- Species having 10 electrons: N3-, O2-, F–, Ne, Na+, Mg2+, Al3+, CH4, NH3, H2O, HF.
- Species having 18 electrons: P3-, S2-, Cl–, Ar, K+, Ca2+.
- Species having 2 electrons: H–, He, Li+, Be2+
- Species having 14 electrons: CO, CN–, N2.
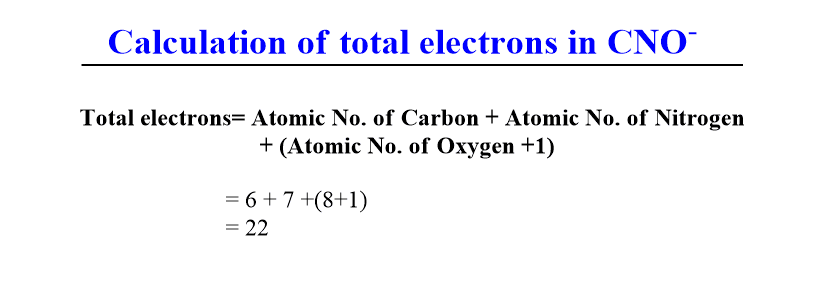
- Species having 22 electrons: N2O, CO2, CNO–.
How to identify isoelectronic species?
It is quite easy to identify isoelectronic species either by counting the total number of electrons or by writing electronic configuration. The electronic configuration of atoms or ions can be easily written but that of molecules is slightly difficult. Thus, I prefer to identify such species by counting electrons. I have tried to present simple way for the calculation of electrons in either atom, ions or molecules below.
- First of all, identify the given species whether an atom or ion or molecule.
- If given species is an atom, its total number of electrons is equal to its atomic number. For example, Argon(Ar) atom has atomic number 18 and a total electron of 18.
- If the given species is a positively charged ion, then the total number of electron=Atomic number- number of positive charges. For example, K+ ion has one positive charge. Thus, the total number of electron= 19-1=18
- If the given species is a negatively charged ion, then the total number of electron= Atomic number + number of negative charge. For example, Cl– ion has one negative charge(it has gained one electron). Thus, the total number of electron= 17+1=18.
- If the given species is a neutral molecule such as AB, the total number of electron= Atomic number of A + Atomic number of B. For example, CO molecule has two atoms viz. one carbon and one oxygen. Thus, total electron= 6+8= 14.
Atomic radius of isoelectronic species
As stated above, these species have the same number of electrons, right? Let’s consider examples of isoelectronic species: S2-, Cl–, K+, Ca2+
Each of these has total number of electrons=18. But these have different atomic number(different number of proton).
S2- ion has 16 protons in its nucleus and 18 electrons in shell whereas Cl– ion has 17 protons and 18 electrons. Now just think, will 16 protons in S2- attract 18 electrons more effectively than 17 protons in Cl– ion?
Its obviously 17 protons(+ve charge) will attract 18 electrons more powerfully. It means effective nuclear charge is greater for Cl– ion since it has more number of protons in nucleus. Therefore, the atomic radius slightly decreases in Cl– than S2-.
Alternatively, we can explain this by calculating the Z/e ratio. Where, Z=atomic number and e= number of electrons. Remember, smaller the value of (Z/e) ratio, larger will be the size(radius).
Z/e for S2-= 16/18= 0.66
Z/e value for Cl– = 17/18= 0.94
Here, S2- have smaller ratio value than Cl–. Hence we can say that, S2- ion has larger radius than Cl–.
Write 4 species that are isoelectronic with the chloride ion Cl–
4 Species that are isoelectronic with Chloride ion(Cl–) are P3-, S2-, Ar, and K+.
Write down 4 species that are isoelectronic with the selenide ion Se2-
Selenide ion (Se2-) has total of 36 electrons and 4 species that are isoelectronic with it are Br–, Kr, Rb+and Sr2+.
Where, Br–=Bromide ion, Kr=Krypton, Rb+=Rubidium ion, Sr2+= Strontium ion.
Write down 4 species that are isoelectronic with Xenon(Xe)
Xenon has total of 54 electrons and 4 species that are isoelectronic with it are Te2-, I–, Cs+ and Ba2+.
Where, Te2-=Telluride ion, I–=Iodide ion, Cs+=Cesium ion and Ba2+= Barium ion.
Which of the following pairs of ions represent isoelectronic species?
- CO2 and NO2
- NH3 and Mg2+
- N2 and CO
- All
The answer of this question is All.
FAQs/MCQs:
Which of these pairs consists of isoelectronic species?
1. Na and Mg2+
2. CN– and S
3. H2 and Li+
4. Ca2+ and K
All of the following species are isoelectronic except
1. N3- and HF
2. P3- and Ca2+
3. CO and CN–
4. CH4 and CNO–






