Table of Contents
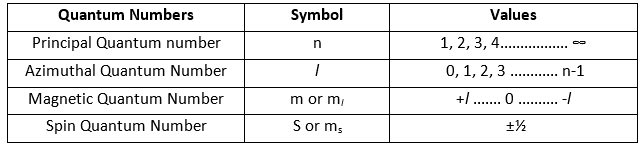
ToggleQuantum numbers are used to describe the position and energy of an electron in an atom. The four quantum numbers that designate an electron in terms of size, shape, and orientation of the orbital in an atom are:
- Principal Quantum Number
- Azimuthal Quantum number
- Magnetic Quantum number
- Spin Quantum Number
Principal Quantum Number (n)
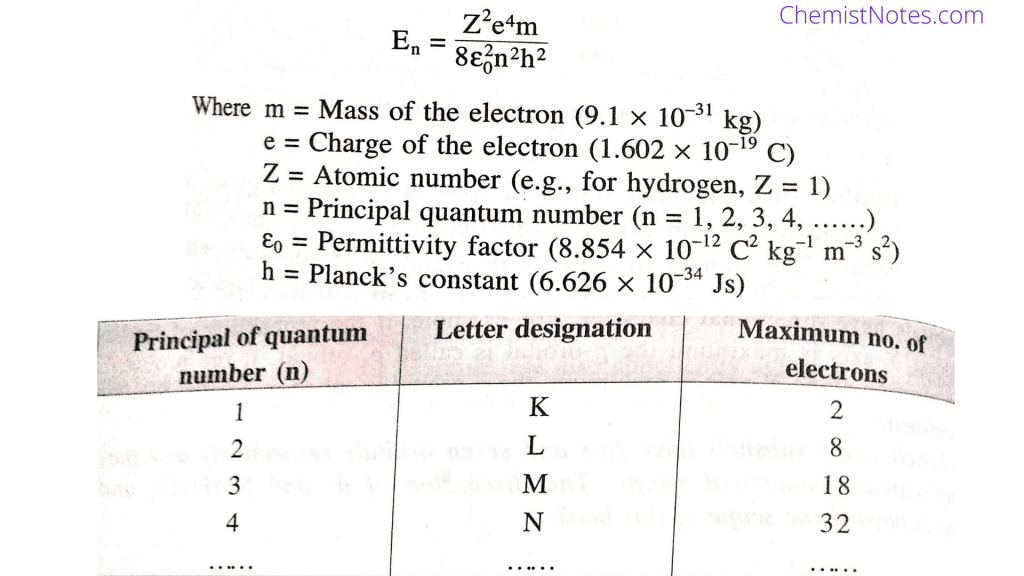
The principal quantum number is represented by ‘n’. It is used to describe the shell or energy level to which the electron belongs. Some of the characteristic features of principal quantum numbers are:
- It can have all non-zero, positive integral value from 1 to ∞ i.e. n = 1, 2, 3, 4 …………….. ∞
- Gives average distance of electron from the nucleus ( r = n2 × 0.529 Å)
- Gives the number of orbital present in a shell (n2).
- Energy of an electron can be calculated using principal quantum number.
- Maximum number of electron that can be accomodated in a shell (2n2) can be known using ‘n’.
Azimuthal Quantum Number (l)
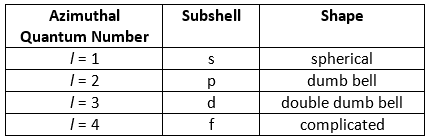
The Azimuthal quantum number is represented by ‘l‘. It represents the angular distribution of electron cloud around the nucleus and is used to describe the shape of orbitals and the angular momentum of an electron. Some of the characteristic features of azimuthal quantum numbers are:
- Represents subshell or sub-energy level
- It’s value varies from 0 to n-1 i.e. l = 0, 1, 2, 3 ………… n-1
- Gives number of subshell present in a shell ( no. of subshell = l).
- Number of electrons that can be accomodated in a subshell can be known using azimuthal quantum number i.e. 4l+2
- Represents number of orbital present in a subshell (2l+1).
- Represents shape of a subshell
Magnetic Quantum Number (m or ml)
The magnetic quantum number is represented by ‘m’ or ‘ml‘ It represents the spatial orientation of atomic orbitals present in a subshell. Some of the characteristic features of magnetic quantum numbers are:
- Represents orientation of electron cloud in a sub-shell, and the number of atomic orbitals in it.
- It’s value ranges from +l to –l i.e. m = +l ……. 0 ………. –l)
Spin Quantum number (s or ms)
The spin quantum number is represented by ‘s’ or ‘ms‘. It describes the direction of the electron spin. An electron may have a spin of either +1/2, represented by↑ i.e. clockwise, or –1/2, represented by ↓ i.e. anticlockwise. Its value doesn’t depend on any other quantum number. Thus, the vale of s/ ms can be described by ±½. The spin quantum number is used to explain whether an atom can generate an electric field or not.
Quantum Numbers Video
FAQs/MCQs
Which characteristic is given by the angular momentum quantum number?
The angular quantum number represents the angular distribution of electron cloud around the nucleus and is used to describe the shape of orbitals and the angular momentum of an electron.
The principal quantum number indicates what property of an electron
The principal quantum number indicates the shell or energy level to which the electron belongs.
What does the angular momentum quantum number determine?
The angular quantum number determines the subshell and its shape.
Which quantum number describes the shape of an orbital?
The azimuthal quantum number/Angular momentum quantum number determines the shape of an orbital
Which quantum number describes the orientation of an orbital?
The magnetic quantum number describes the orientation of an orbital.
Who proposed azimuthal quantum number?
Sommerfeld proposed azimuthal quantum number.
significance of magnetic quantum number
It represents the spatial orientation of atomic orbitals present in a subshell.
value of magnetic quantum number for p orbitals
The value of magnetic quantum number for p orbitals is +1, 0, -1.






