Table of Contents
TogglePrinciples of VSEPR theory, its definition, some examples, limitations, and advantages of it have been discussed in this blog.
VSEPR theory is also called the Valence shell electron pair repulsion theory. It is a simple theory that predicts and explains the shape and bond angles in covalent molecules and ions. This theory was proposed by Sedgwick and Powell in 1940 at first and then developed by Gillespie and Nyholm in 1957.
VSEPR Theory Definition
VSEPR Theory is a model for predicting the shape and bond angles of simple covalent molecules and ions. This theory allows us to determine the shape of a number of covalent molecules.
Main features or principles of VSEPR Theory
The postulates of this theory are given below:
- The total number of electron pairs present in the valence shell of the central atom determines the shape of the molecule. These electron pairs are arranged around the central atom in the space in such a way that there is minimum electrostatic repulsion between them and hence shape is determined.
- If the central atom has lone pairs(lp) along with the bond pairs(bp), the ideal shape is distorted to give a different bond angle than expected. This is because a lone pair occupies more space around the central atom than the bond pairs since the lone pair is attracted by one nucleus and the bond pair is shared between two nuclei. The order of repulsion is: lp-lp>lp-bp>bp-bp
- In isostructural molecules, the electronegativity of central atom affect the bond angle. As the electronegativity of the central atom increases,the bond pairs of electrons are pulled more towards the central atom due to which repulsion between bond pairs increase and hence bond angle increases.
As the electronegativity of atoms bonded to central atoms increases the bond pairs of electrons are pulled towards electronegative atoms. Hence repulsion between bond pairs and bond angle decreases.
4. The double or triple bond is considered as one electron pair. The order of repulsion is given as:
Single bond<double bond< triple bond
Valence Shell Electron Repulsion Theory Examples
Here are some examples of covalent molecules which are described according to Valence shell electron pair repulsion theory.
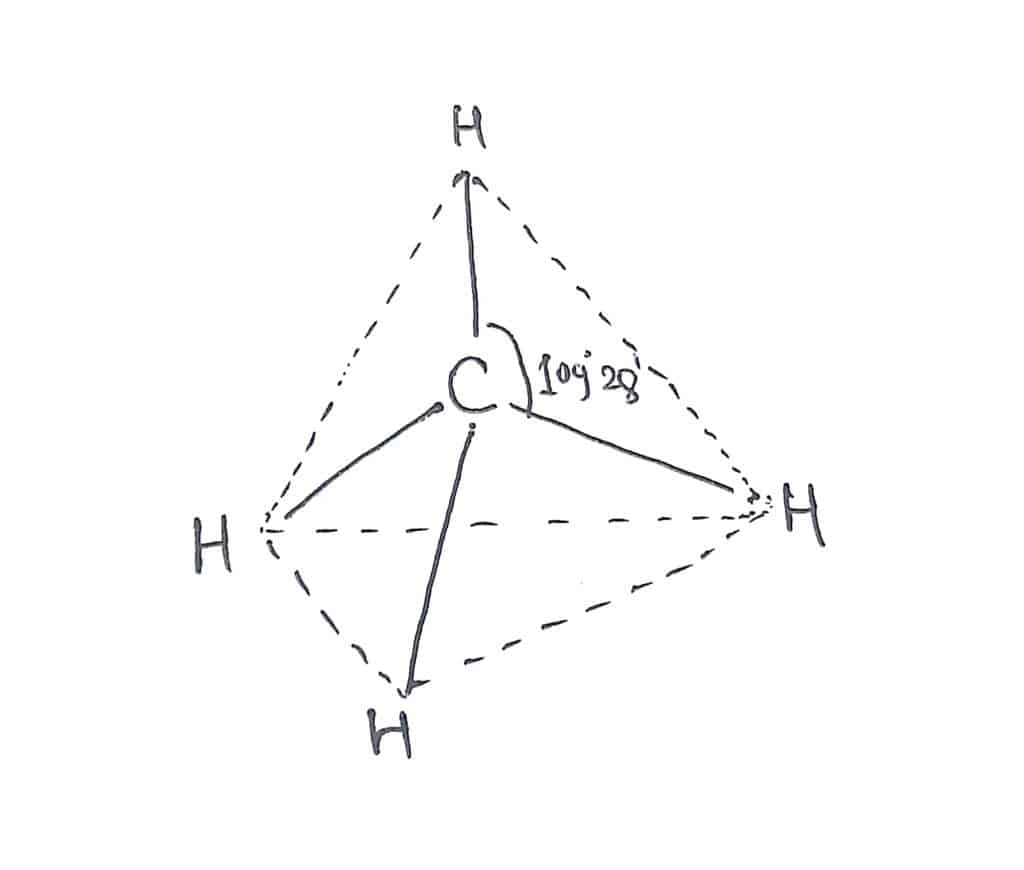
1.Methane
In methane(CH4), there are four bonded pairs of electrons around the central carbon atom. There is no lone pair of electrons. Therefore, these four bonded pairs are arranged in a such way to minimize the repulsion between them, and hence tetrahedral geometry with bond angle 109° 28′ results.
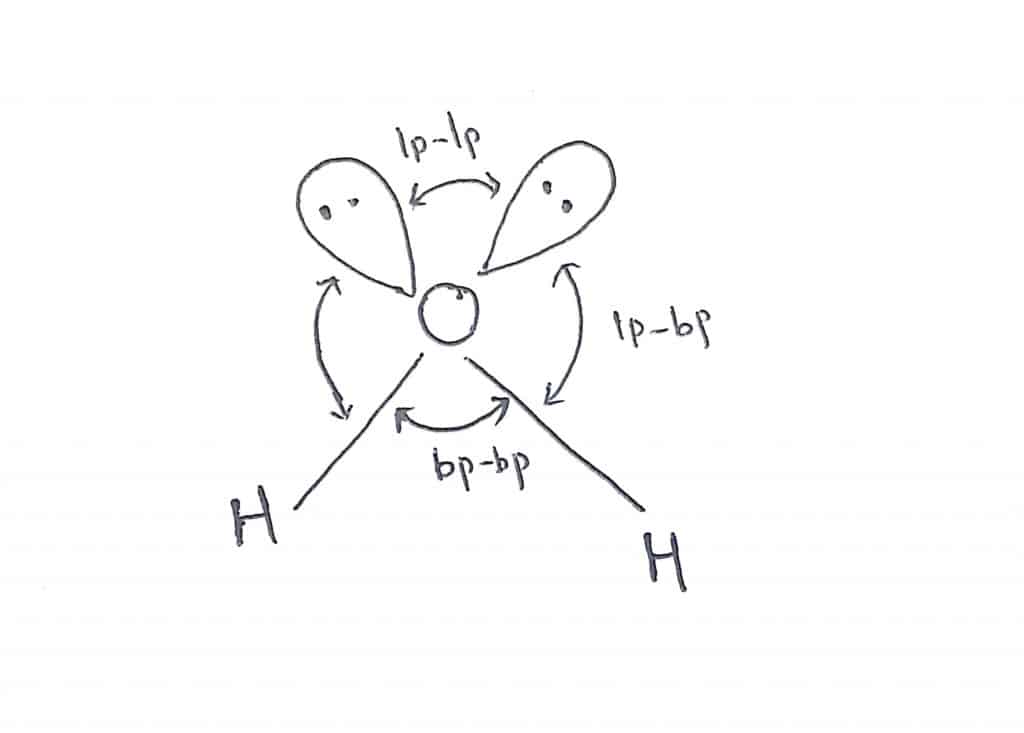
2.Water
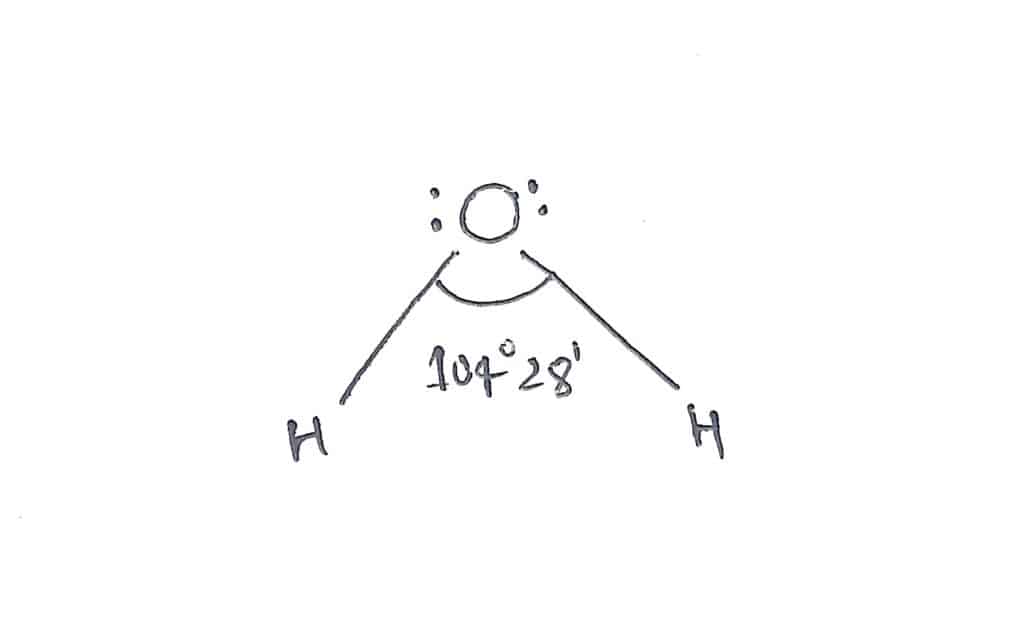
In water(H2O), there are four electrons pairs( two bond pairs + two lone pairs) around the central oxygen atom. Therefore, the predicted geometry is tetrahedral but due to the presence of two lone pairs of electrons, the actual geometry becomes bent or angular with bond angle 104°28′.
VSEPR Theory Chart/Table
The shape of molecules on the basis of this theory is tabulated below:
| No.of bond pairs | Expected Geometry | Bond angle | Example |
| 2 | Linear | 180° | BeCl2, BeF2 |
| 3 | Trigonal planar | 120° | BF3,BCl3 |
| 4 | Tetrahedral | 109°28′ | CH4, CCl4 |
| 5 | Trigonal bipyramidal | 90°,120° | PCl5 |
| 6 | Octahedral | 90° | SF6 |
| 7 | Pentagonal bipyramidal | 72°,90° | IF7 |
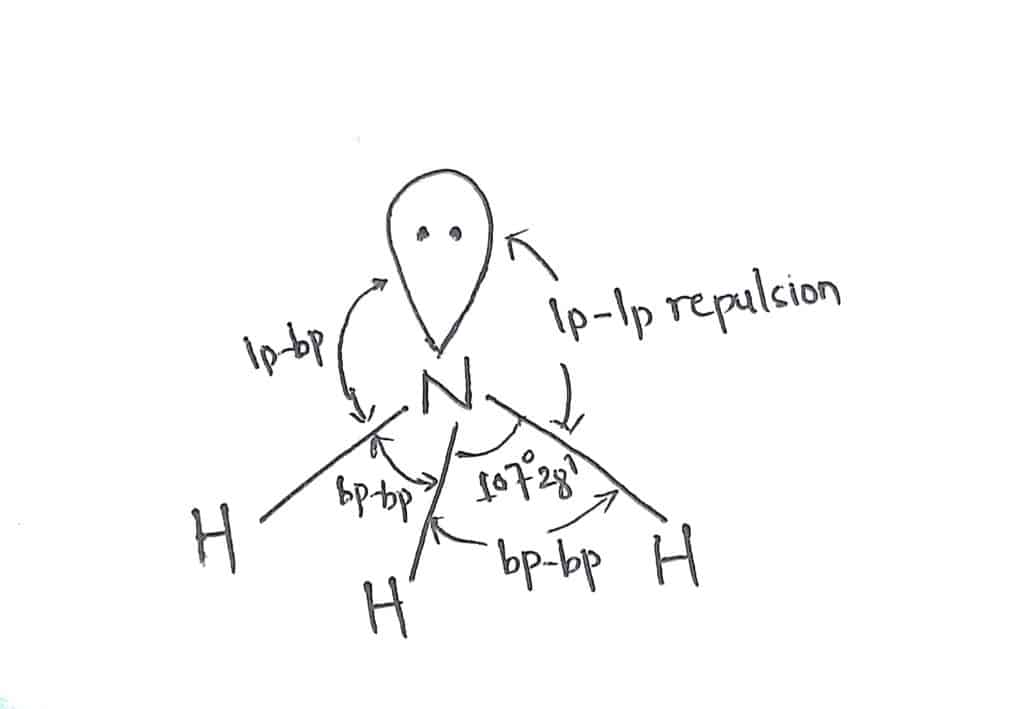
Shape of NH3 according to VSEPR Theory
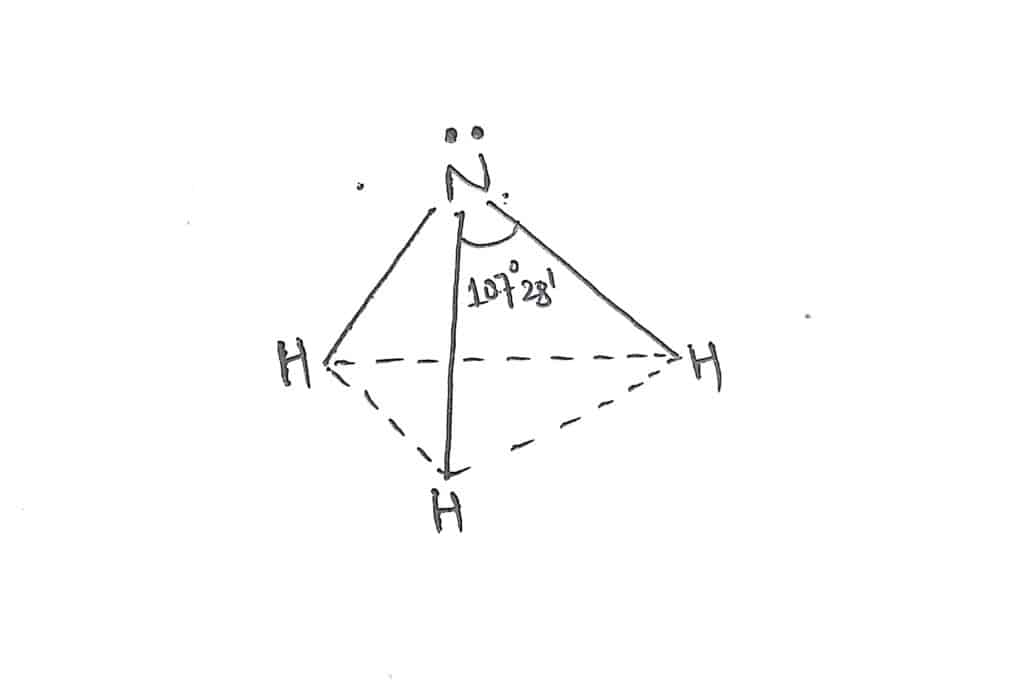
In ammonia, there are four electrons pairs(Three bond pair+ one lone pair) around the central nitrogen atom. The expected geometry of NH3 is Tetrahedral with a bond angle of 109° 28′.But, due to the presence of one lone pair of electrons around the central atom, it exerts greater repulsion than the bond pair. Therefore, it has the actual shape of trigonal pyramidal with bond angle 107°28′.
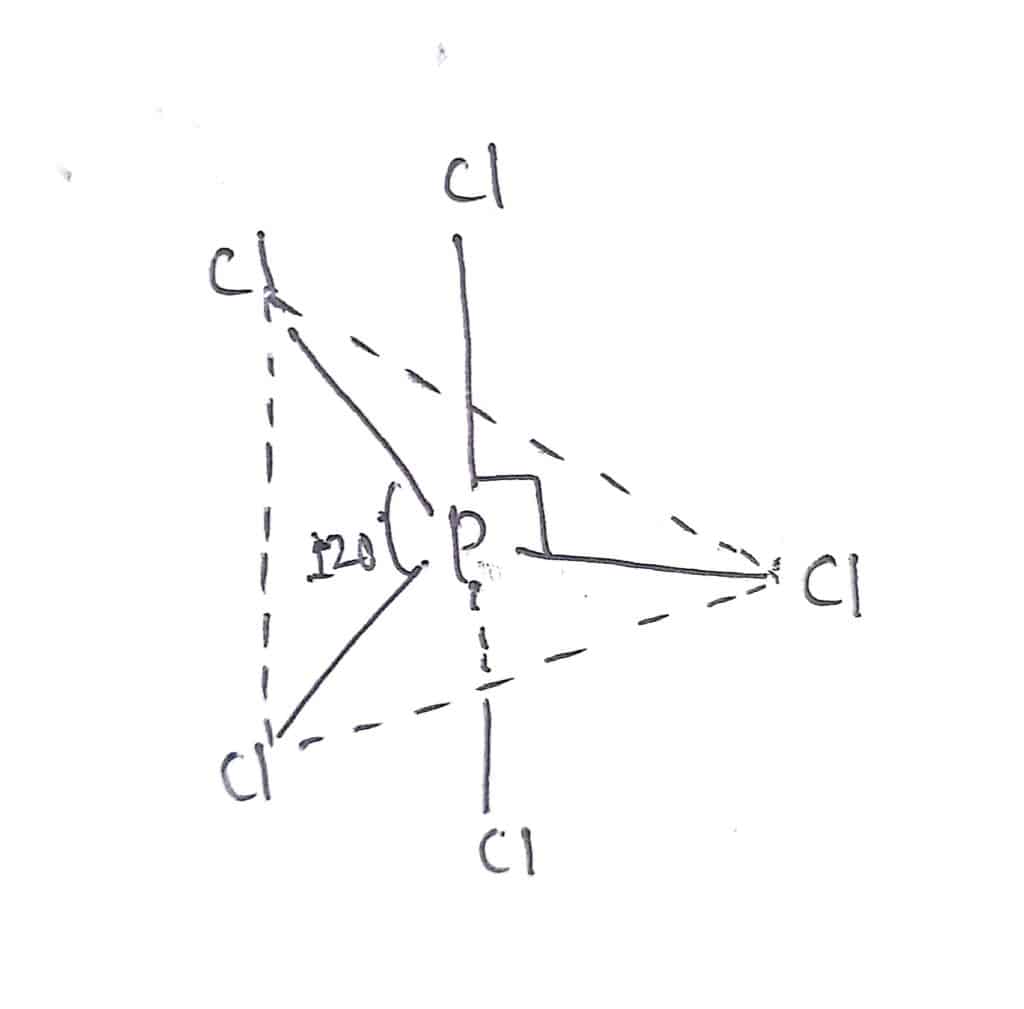
Shape of PCl5
In Phosphorous pentachloride, there are five electron pairs around the central phosphorus atom. There are no lone pairs of electrons in PCl5. The repulsion of five electrons pairs is minimum when they are arranged in trigonal bipyramidal shape. Therefore, PCl5 has a trigonal bipyramidal shape.
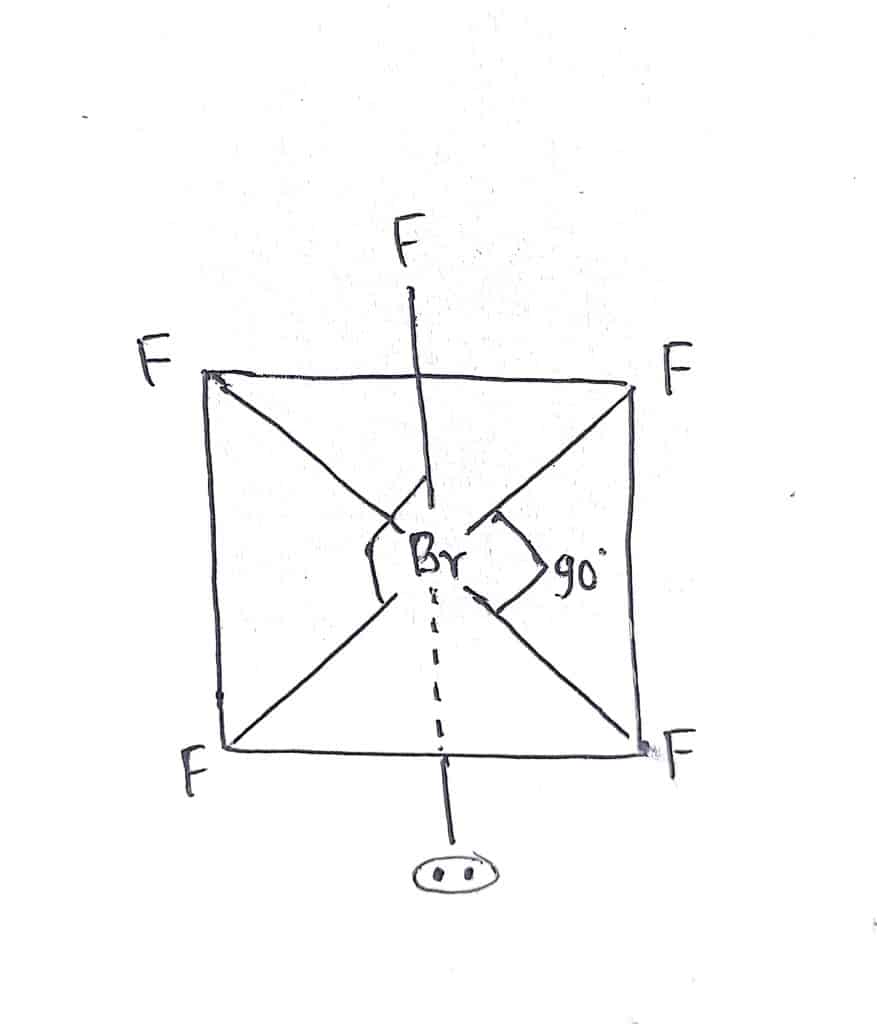
Shape of BrF5
In Bromine pentafluoride, there are six electron pairs(5 bond pairs + one lone pair) around the central Bromine atom. The expected geometry of BrF5 is octahedral but due to the presence of one lone pair, it exerts greater repulsion than bond pairs. Therefore, it has the actual shape of square pyramidal. The lone pair of electrons occupies an axial position as shown in the figure.
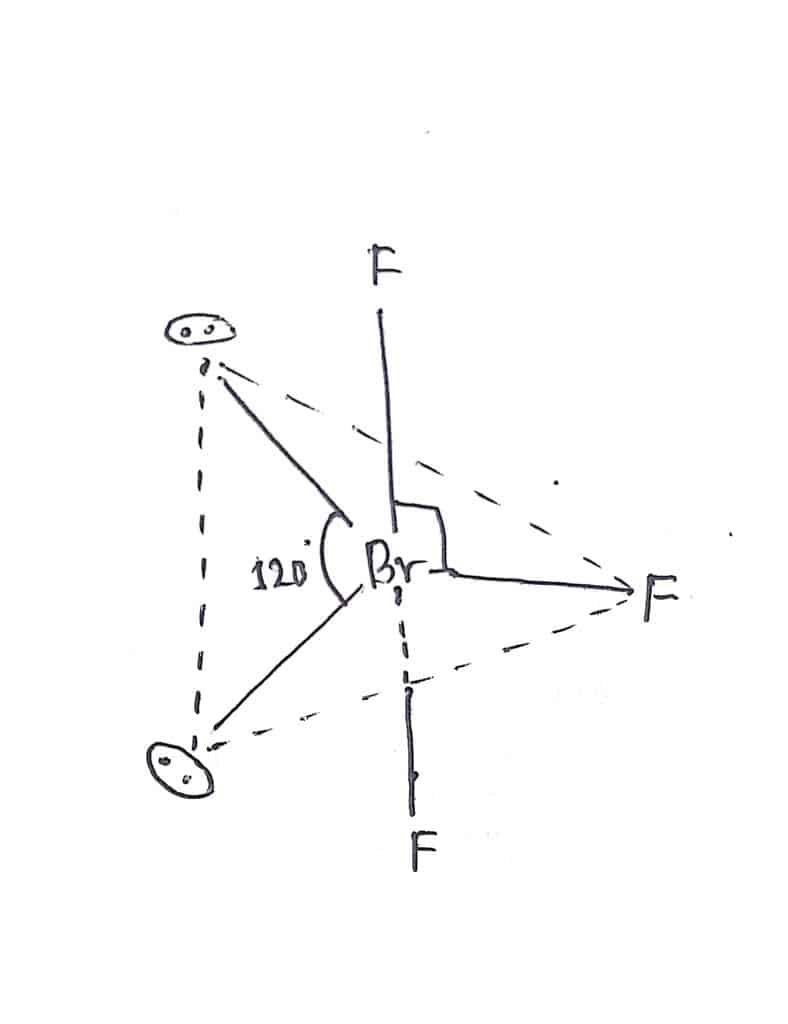
Shape of BrF3 using Valence shell electron repulsion theory
In Bromine trifluoride, there is a total of five electron pairs(3 bond pairs + 2 lone pairs) around the central Bromine atom. The expected geometry of BrF3 is trigonal bipyramidal. Due to the presence of 2 lone pairs, the actual shape of the molecule becomes T-shape. The lone pairs occupy equatorial position as shown in the figure below:
Advantages of VSEPR Theory
Some advantages of this theory are listed below:
- It can be used to predict the geometry of covalent molecules and ions
- It can be used to predict the number of regions containing high electron density around central atom in the molecules.
Limitations of VSEPR theory
Some of the limitations of this theory are listed below:
- It fails to explain the geometry of isoelectronic species.
- It doesn’t describe the shape of transition metal compounds
- It doesn’t differentiate between single, double and triple bond.
- It doesn’t explains the bond strength.
Difference between Valence shell electron repulsion theory and Valence bond theory
The differences between the VSEPR and valence bond theory is given below:
| VSEPR Theory | Valence bond Theory |
| The VSEPR theory predicts the geometry/shape of covalent molecules. | Valence bond theory explains the formation of chemical bond in covalent molecules. |
| It is based on the repulsion between bond pairs and lone pairs of electrons. | It is based on the overlap of atomic orbitals to form a chemical bond. |
| This theory does not explain orbitals present in atoms of molecules. | This theory explains orbitals present in atoms of molecules. |
| It does not give an idea about the type of bond present in the molecules. | It gives an idea about the type of bond present in the molecules. |
VSEPR Theory Video
FAQs/MCQs:
What does the VSEPR theory predict?
The VSEPR theory predicts the geometry of simple covalent molecules with bond angles.
What are repelled in VSEPR theory?
Electron pairs (bond pairs+ lone pairs) are repelled in VSEPR theory. The order of repulsion is:
Lone pair-Lone pair> Lone pair-Bond pair> Bond pair-Bond pair.
how does the VSEPR theory explain molecular shape?
The VSEPR theory explains molecular shape on the basis of a number of electron pairs present around the central atom.
what does the vsepr theory describe?
The vsepr theory describe the geometry of covalent molecules.
what is described by the vsepr theory?
Geometry/shape and bond angle of covalent molecule is described by the VSEPR theory.
what is vsepr theory in chemistry?
VSEPR Theory is a model for predicting the shape and bond angles in simple covalent molecules and ions.
what do scientists use VSEPR theory for?
Scientists use the VSEPR theory for predicting the geometry of Covalent molecules.
what does the VSEPR theory tell about a molecule?
The VSEPR theory tells about the shape, geometry, and bond angle of a molecules.






