Table of Contents
ToggleBuckminster Fullerene (C60) is the most common fullerene, which is the pure crystalline allotropic form of carbon. In 1996, Richard Smalley, Robert Curl, and Harold Kroto shared the Nobel Prize in Chemistry for discovering a new carbon allotrope with the formula C60 buckminsterfullerene. It consists of 60 carbon atoms with the geometry of an icosahedron, having 20 hexagons and 12 pentagons, and is nearly spherical in shape (like a soccer ball). C60 is one of the most strained molecules but exhibits great kinetic stability.
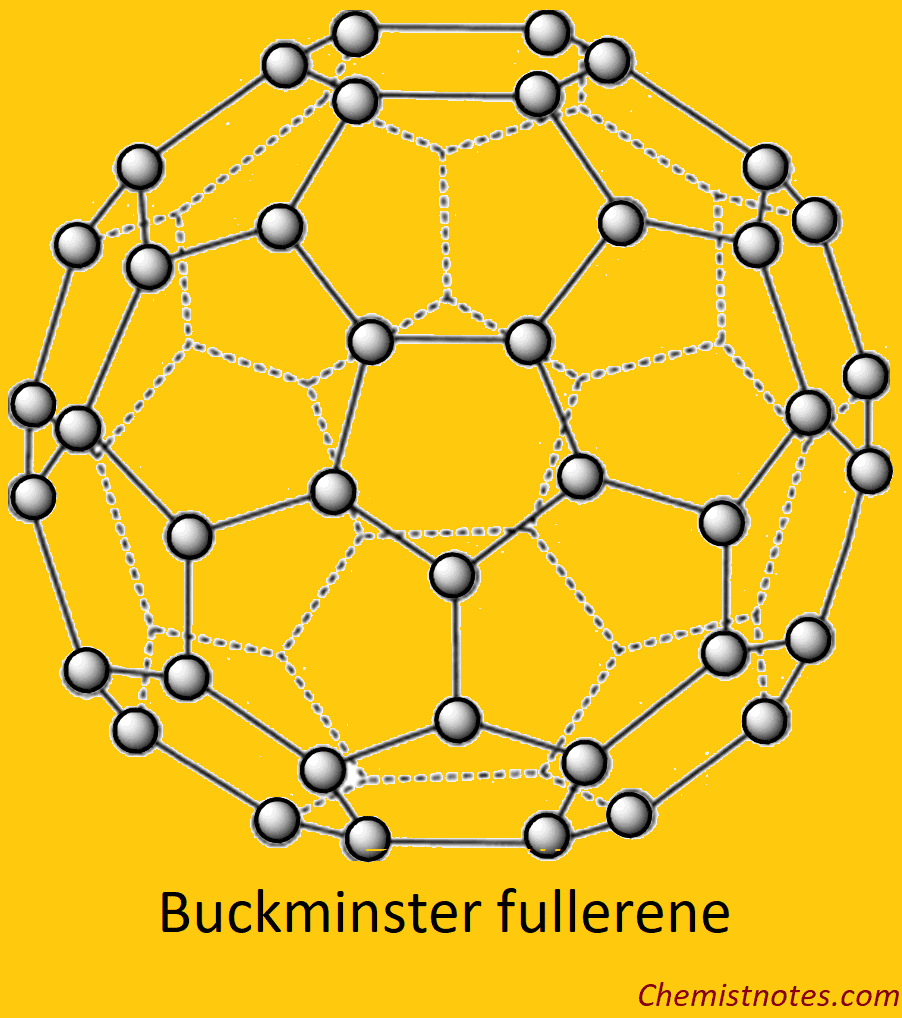
The Structure of Buckminster Fullerene
Buckminsterfullerene is a remarkably stable cluster compound containing 60 carbon atoms. Concerning the question of what kind of carbon atom structure might give rise to the super stable species, it is suggested to be truncated icosahedral; a polygon with 60 vertices and 32 faces, 12 of which are pentagons and 20 hexagons. The C60 molecules that result when the carbon atoms are placed in each vertex are satisfied by two single and double bonds with many resonating structures and thus appear to be aromatic. All the 60 carbon atoms are equivalent and give rise to a single 13C NMR resonance. The outer diameter of the C60 molecule is 7.10 Å and its van der Waals separation is 2.9 Å so that the nearest neighbor distance (effective diameter) in a solid is 10.0 Å. The bonds shared by a five-membered and a six-membered ring are 1.45 Å long, while those between two adjacent six-membered rings are 1.40 Å long.
Properties of Buckminster Fullerene
Buckminsterfullerene or C60 or buckyballs has certain unique properties:
- It is very tough and it can accelerate to 1500 miles per hour and can be slammed against a hard surface without getting damage.
- It can compress to lose 30 % of its volume without destroying in carbon cage structure.
- It is thermally stable and it can be sublimed at 600oC under a vacuum.
- It exists as a discrete molecule, unlike the other two carbon allotropes graphite and diamond, from the lattice.
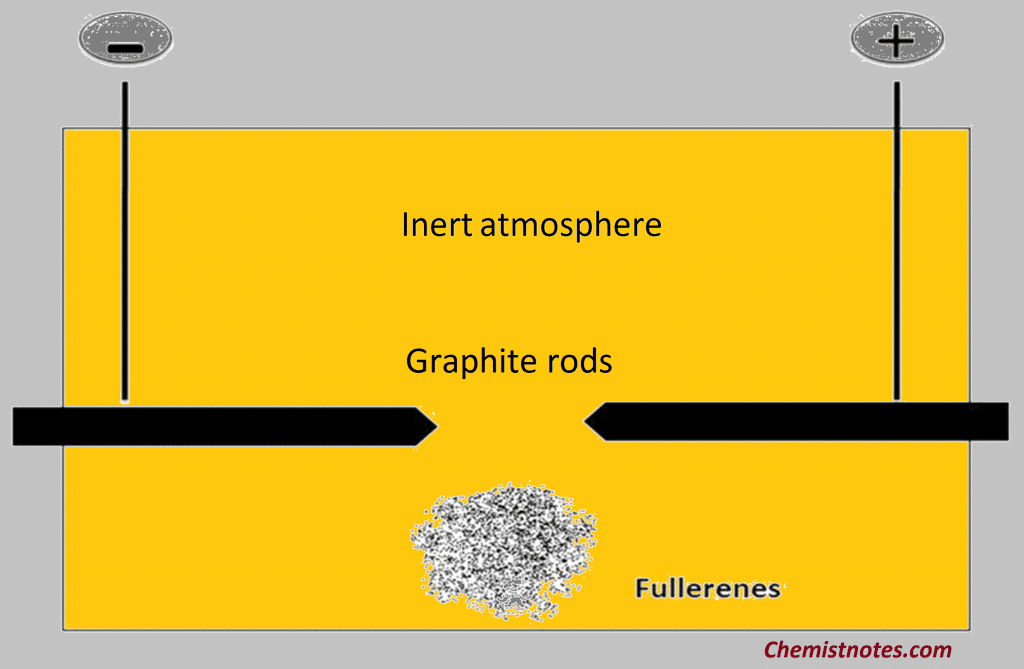
Preparation of Buckminster Fullerene
The Buckminster Fullerene was discovered in 1985 using pulse laser vaporization of graphite and was detected by mass spectroscopy. When an electric ark is struck between carbon electrodes in an inert atmosphere, a large quantity of soot is formed together with significant quantities of C60 and much smaller quantities of related fullerenes such as C70, C76, and C84. The fullerenes can be dissolved in a hydrocarbon or halogenated hydrocarbon and separated by chromatography on an alumina column. The structure of C60 has been determined by x-ray crystallography on the solid at low temperatures and electron diffraction in the gas phase. The molecule consists of five and six-membered carbon rings, and the overall symmetry is icosahedral in the gas phase.
Applications
- The fullerene C60 nano-oil is proposed as a good lubricant to enhance the performance of residential refrigerator compression.
- The compound C60 is not itself a superconductor, but when alkali metals are added it becomes superconducting. The compound of fullerene with alkali metals such as K3C60 acts s a conductor at room temperature and a superconductor below 18K.
- Buckminsterfullerenes can be used as optical limiters due to their ability to decrease the transmittance of light. They are thus incredibly useful for creating optical sensors and safety glasses.
- Fullerenes are also used in data storage devices, solar cells, fuel cells, and telecommunication devices.
- Buckminsterfullerene finds exciting applications in medicine. As the C60 molecule has approximately the same radius as the cylinder of HIV protease enzyme and its derivatives are primarily hydrophobic. So, C60 protects the active site of the HIV protease enzyme by the attacking species.
- The special properties of fullerene have promise for certain types of drug design. The small size, spherical shape, and hollow interior all provide therapeutic opportunities.
- Moreover, a cage of 60 carbon atoms has places at which chemical groups can attach in almost any configuration. Such opportunity has led to the development of not only drug candidates treating HIV, Cancer, and neurological disorders but also new diagnostic tools.
FAQs
Does Buckminster fullerene conduct electricity?
The compound C60 is not itself a superconductor, but when alkali metals are added it becomes superconducting.
What are buckminster fullerenes?
Buckminster Fullerene (C60) is the most common fullerene, which is the pure crystalline allotropic form of carbon.
What are buckminster fullerenes used for?
Buckminster fullerenes are used as a fine lubricant, in various medical fields, as superconductors, and in various cells and devices.
Are there double bonds in buckminsterfullerene?
Yes, all carbon atoms in buckminsterfullerene are part of one double bond.






