Table of Contents
ToggleChemical oxygen demand is an index of pollution that measures the effect of pollutants on dissolved oxygen. In the COD test, an oxidant other than O2 is used to degrade the pollutants in the water sample. The amount of the oxidant consumed is experimentally measured to calculate the equivalent amount of oxygen required by the wastewater for the degradation of the pollutants. Acidified Cr2O7, which is a stronger oxidant than O2, is commonly used for this test.
The basic equation involved in the COD test is the following
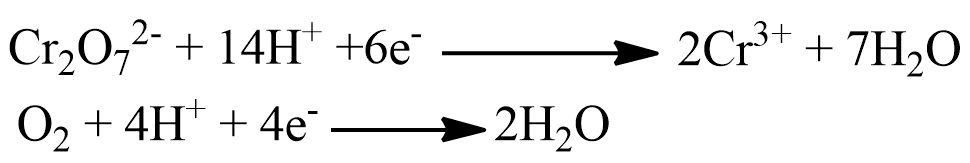
Therefore, 1 mole of Cr2O72- consumed = 6/4 = 1.5 moles of oxygen used.
The COD evaluation is carried out by adding a known excess of Cr2O72- (aq) to an aliquot of the water sample and then estimating the unused Cr2O72- by titrating it with Fe2+ (aq). Neither O2 nor Cr2O72- can oxidise certain pollutants such as alkanes and aromatic hydrocarbons.
When a water sample is highly polluted with organic waste, the oxygen demand will exceed the maximum equilibrium solubility of O2 in water. Such a water sample may not contain any dissolved O2.
Determination of chemical oxygen demand
The chemical oxygen demand (COD) represents the amount of oxygen in milligrams required to oxidised all the organic pollutants present in water to carbon dioxide and water. This oxidation can be represented by the general equation shown below.
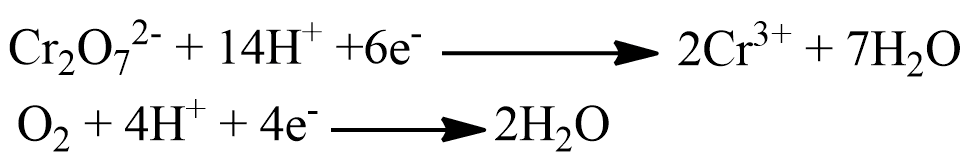
Principle
The water sample is boiled with potassium dichromate in a strongly acidic medium under reflux. The organic compounds present in the sample are oxidized by the dichromate. This oxidation is catalyzed by silver sulfate. The catalyst is required, especially to oxidize low molecular weight fatty acids. The oxidation by the acidified dichromate can be represented by the following equation:

This method has been found successful when the proportions and types of materials in a wastewater remain relatively constant.
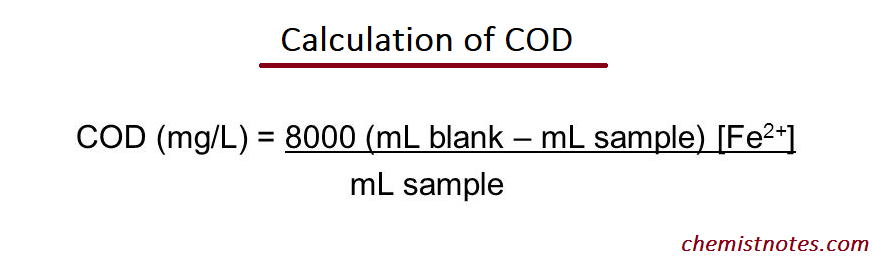
Application of COD
- COD is frequently used to quantify contaminants in water, wastewater, and aqueous hazardous wastes.
- All organic pollutants, even those that are not biodegradable, can be measured using COD.
- The results of the COD test can then be used to estimate the BOD of a specific sample.
- The COD test can be used to determine the strength of wastes that are too toxic to be tested through using BOD test.
Difference Between BOD and COD
| Biological oxygen demand (BOD) | Chemical oxygen demand (COD) |
| BOD may be defined as the amount of oxygen used for biochemical oxidation by microorganisms in a unit volume of water. The test has been developed for 5 days at 20oC. | It may be defined as the amount of oxygen required by organic matter in a sample of water for its oxidation by a strong oxidizing agent and is expressed as ppm of oxygen taken from a solution of K2Cr2O7 in two forms. |
| The test has been developed for 5 days at 20oC. | The COD test only requires 2-3 hours. |
| BOD value approximates the amount of oxidizable organic matter. Hence, it is used as a measure of the degree of water pollution and waste strength. | This value has been a poor measure of the strength of organic matter as oxygen also gets consumed in the oxidation of inorganic matter such as nitrates, sulfates, and reduced metal ions, and also that some organic materials like benzene pyridine and a few other cyclic organic compounds do not get oxidized by this test. |
| Types of microorganisms, pH, presence of toxins, some reduced mineral matter, and nitrification process have been the important factors that influence the BOD test. | The presence of toxins and other unfavorable conditions for the growth of microorganisms are not able to affect the COD values. |
FAQs
What is chemical oxygen demand?
Chemical oxygen demand is an index of pollution that measures the effect of pollutants on dissolved oxygen.






