Table of Contents
ToggleProteins are the most prevalent organic molecules in living beings. They serve as the foundation for the development and functioning of life and makeup 50% of each cell’s dry weight. Proteins are an important macronutrient essential for survival.
Protein Definition
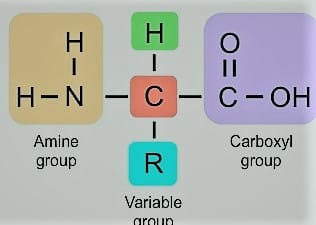
Proteins are large, complex molecules that are essential to numerous body systems. They perform the majority of their work within cells and are necessary for the development, maintenance, and control of the body’s tissues and organs. Proteins are composed of hundreds to hundreds of amino acids with each other in a long chain-like structure. There are generally twenty types of amino acids including non-essential and essential to make proteins.
Proteins generally consist of five major elements as base components such as carbon, hydrogen, oxygen, nitrogen, and sulfur. There are various important functions of proteins which are discussed below: They perform many specialized and essential functions grouped as structural and dynamic functions.
Function of proteins
- They are responsible for the structure and strength of the body.
- These structural functions included collagen and elastin found in bone, matrix, and vascular systems.
- They include proteins acting as enzymes, hormones, blood clotting factors, and membrane receptors.
- They also act as working horses of cells.
Structure of Proteins
Proteins are the polymers of alpha amino acids. They are classified into four categories:
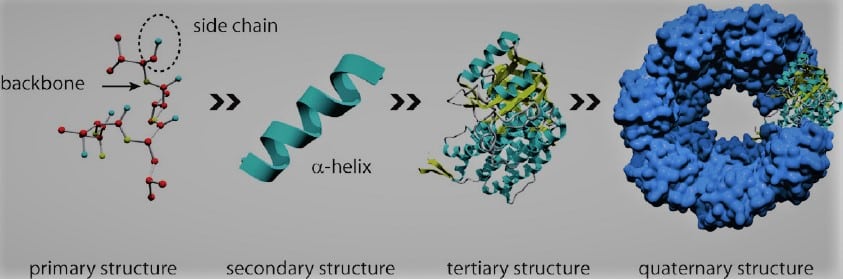
1. Primary structure
The fundamental structure of a protein is only the arrangement of the amino acids in a polypeptide chain. Insulin, for instance, contains two polypeptide chains. They are the linear sequence of amino acids forming the backbone of proteins.
2. Secondary structure
They are formed by the spatial arrangement of protein by twisting the polypeptide chain. Secondary structure, the next level of protein structure, describes the local folded shapes that develop within a polypeptide as a result of interactions between the atoms in the backbone.
3. Tertiary structure
They are the three-dimensional structure of a functional protein. A polypeptide’s overall three-dimensional structure is referred to as its tertiary structure. The interactions between the R groups of the amino acids that make up the protein are principally responsible for the tertiary structure.
4. Quaternary structure
They are formed by two or more polypeptide chains referred to as subunits which beings arranged to form the quaternary structure of the protein. Many proteins only have three levels of structure and are composed of a single polypeptide chain. Nevertheless, some proteins are composed of several polypeptide chains, also referred to as subunits. These component parts combine to form the protein’s quaternary structure.
Classification of Proteins
Proteins are classified generally based on the basis of their function, chemical nature, solubility properties, and nutritional importance which are explained below:
1. Classification based on Solubility and composition
On the basis of solubility and composition, proteins are classified into three types, they are simple, conjugated, and derived proteins.
- Simple protein: When simple proteins are hydrolyzed, only amino acids are produced. These proteins are further divided into groups according to how easily they dissolve in various solvents and how easily they coagulate under heat:
- Albumins: They are readily soluble in dilute acids, water, and alkalies. They are easily coagulated by heat along with a deficient glycine in them.
- Globulins: They are sparingly or insoluble in water. They are deficient in methionine.
- Prolamins: They are insoluble in water but soluble in aqueous alcohol at 80-85%. They are deficient in lysine.
- Glutelins: They are insoluble in water but soluble in acids and alkalies. They are plant proteins.
- Histones: Histones are a type of tiny, stable protein. They have a considerable proportion of the fundamental amino acid histidine. They are insoluble in ammonium hydroxide but soluble in water. Heat does not easily cause them to coagulate. They can be found in nucleoproteins and hemoglobin.
- Albuminoids: These exhibit high stability and are insoluble in salt and water solutions. Because they resemble albumin and globulins in many ways, these are known as albuminoids. They have a high level of proteolytic enzyme resistance.
2. Conjugated protein: They are generally simple proteins composed of non- proteins structures called prosthetic groups. The subclassification of conjugated proteins is based on the characteristics of the non-protein or prosthetic groups.
- Nucleoproteins: Protamines or histones are simple, basic proteins that are combined with nucleic acids as a prosthetic group to form nucleoproteins, which are crucial components of chromatin and nuclei.
- Chromoprotein: These are proteins including cytochrome, flavoprotein, and hemoglobin that contain colored prosthetic groups.
- Lipoproteins: These are lipid-conjugated proteins that include cholesterol, neutral fat, and phospholipids.
3. Derived proteins: These are proteins that have undergone partial to complete hydrolysis by the action of acids, alkalies, or enzymes from simple or conjugated proteins. They consist of primary-derived proteins and secondary-derived proteins, two different types of derivatives.
2. Classification based on function
- Catalytic proteins: enzymes
The potential of these proteins to serve as biocatalysts within living cells is one of their most noticeable distinguishing characteristics. Enzymes are what are used as biocatalysts. The most prevalent class is enzymes. There are more than 2000 different types of known enzymes, each of which catalyzes a distinct type of reaction.
2. Regulatory protein: Hormones
These are polypeptides and tiny proteins that are present in the animal kingdom in comparatively lower amounts but have a crucial regulatory role in preserving order in complicated metabolic reactions, such as growth hormone, insulin, and others.
3. Protective protein: Antibody
These proteins serve as defensive mechanisms. These proteins mix with other chemicals including foreign proteins to combat specific disorders. such as immunoglobulin. In reaction to foreign molecules known as antigens, the spleen and lymphatic cells create these proteins.
4. Storage protein:
Storage proteins are a prominent family of proteins that serve as a supply of critical amino acids that humans are unable to synthesize and serve as building blocks for the developing embryo. Globulins and prolamins in grains make up the majority of the storage protein in pulses. Glutelins are the main storage protein in rice.
Albumin in eggs and casein in milk are also storage proteins.
3. Classification based on shape and size
On the basis of shape and size, proteins are divided into globular, and fibrous proteins.
Most globular proteins, including enzymes, hormones, and antibodies, are water-soluble and delicate by nature. Fibrous proteins are strong and insoluble in water. They are employed to create a number of substances that support and protect particular tissues, such as keratin, skin, hair, and nails.
Denaturation of proteins
Protein denaturation is a biological phenomenon in which a protein loses its natural form as a result of weak chemical connections and interactions being broken or disrupted, rendering the protein physiologically inactive. It is the process by which correctly folded proteins produced under physiological conditions change into an unfolded proteins when produced under non-physiological situations. Proteins can undergo denaturation under a variety of chemical and physiological circumstances.
Reversible or irreversible denaturation is a possibility. Denaturation often occurs when a protein is exposed to outside forces such as heat, radiation, inorganic solutes, organic solvents, acids, or bases. In other words: Any modification to a protein molecule’s secondary, tertiary, or quaternary structure that causes the breakdown of covalent bonds is known as denaturation, and it is a biochemical process.
Method of denaturation by denaturants
There are various agents and method that causes the denaturation of proteins:
- Heat/ High temperature
- Pressure
- Low temperature
- Irradiation
- Sound waves
- Surface forces
Protein hydrolysis
Peptide bonds are broken during protein hydrolysis, a process carried out at high temperatures and in an acidic environment to produce residues of free amino acids. A further amino acid analysis of the resultant hydrolysates can be used to determine the protein content and amino acid composition.
References
http://eagri.org › eagri50 › BIC101 › pdf › lec12
MCQs/FAQs
What are proteins and examples?
Proteins are organic molecules that are present in a living organism. They serve a wide range of functions including organization, transportation, and defense. Proteins are composed of amino acid chains, and structure levels are up to four. Certain specific protein examples include collagen and insulin.
What are the sources of protein?
Plant-based foods (fruits, vegetables, grains, nuts, and seeds) frequently lack one or more essential amino acids, but animal-based foods (meat, chicken, fish, eggs, and dairy products) are frequently good sources of complete protein.
What is the structural classification of protein?
They are primary, secondary, tertiary, and quaternary structures.
Who discovered protein?
The protein was discovered by Dutch scientist Gerardus Johannes Mulder, and the name was given by Swedish chemist Jons Jacob Berzelius in 1838.
How much protein does the body need every day?
Protein plays a crucial role in the health of human bodies. The average amount of weight required by the human body to perform its functions is 0.8 grams.