Table of Contents
ToggleThe properties of alpha beta and gamma rays were observed and discovered by Rutherford by passing the radiations from radioactive substances through a strong electric field between oppositely charged parallel plates are well explained. The following points were concluded by placing uranium mineral in a lead box and passing emitted radiations between plates as shown in the figure below:
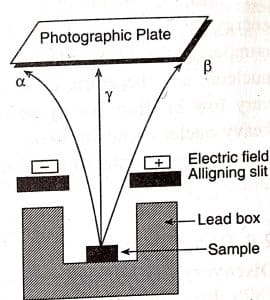
- The radiations bending towards the negative plate (cathode) show that these radiations are carrying a positive charge and are named alpha rays.
- The beta rays or beta particles are radiations that carry a negative charge and are deflected in the direction of the positive plate (anode). Since these rays are deflected to a much larger degree than alpha rays, it can be assumed that beta rays are lighter.
- Gamma rays are the third category of radiations, which are neutral in nature and are not deflected even by a strong electric or magnetic field.
It is found that the radioactivity of an element is independent of its physical state, its chemical environment, or temperature, suggesting that it is a property of the nucleus. Radioactivity is the phenomenon of spontaneous disintegration of certain nuclei into another element with the emission of invisible active radiation.
Properties of alpha beta and gamma rays
The properties of each of these three kinds of radiations are briefly discussed below:
Properties of alpha rays (α-rays)
- They consist of α-particles with two units positive charge and with mass four times that of hydrogen.
- The velocity of α-ray ranges from 1.4 × 107 cm/sec to 1.7 × 107 cm/sec or is nearly (1/10)th of that of light.
- They possess high ionization strength, which enables them to cause strong ionization in the gas that α-ray passed through.
- They have the least penetrating power because of their larger size.
- They are deflected by electric and magnetic fields.
- When α-ray from a radioactive substance collides with a screen made of a fluorescent material, such as zinc sulfide, it can emit light.
- They have the ability to produce a heating effect.
Properties of beta rays (β-rays)
- They consist of negatively charged particles carrying unit negative charge and charge to mass ratio identical to an electron.
- The velocity of β-ray is greater than α-ray and is almost equal to the velocity of light.
- Since β-rays are lighter than α-rays, it possesses lower kinetic energy, thus resulting in lesser ionization power.
- They have high penetration power which is due to their high velocity.
- They are also deflected by electric and magnetic fields.
- They have a higher photoelectric effect in comparison to α-particles.
Properties of gamma rays (γ-rays)
- They are electromagnetic radiation that does not possess any charge and mass.
- The velocity of γ-ray is the same as that of the velocity of light.
- They are not deflected by electric and magnetic fields.
- They have relatively low ionizing power.
- They possess high penetration power and hence can penetrate through an aluminum sheet of nearly 100 cm thickness.
- They have a photographic effect as well as a fluorescence effect.
Difference between alpha beta and gamma rays
| Characteristics | α-rays | β-rays | γ-rays |
| Charge and mass | carries two unit positive charge and four unit mass. | carries one unit negative charge and no mass. | carries neither charge nor mass. |
| Representation | represented as helium nucleus or helium ions ( 24He or He++). | represented as an electron (-1eo) | represented as 00γ |
| Velocity | (1/10)th of the velocity of light | 2.36 to 2.83 × 108 m/sec | same as that of light (3 × 108 m/sec) |
| Action of electric field | deviated towards cathode | deviated towards anode | not deviated |
| Ionizing power | Very high | less (1/100 times) than that of α-rays | very low |
| Penetrating power | Low | 100 times high that of α-rays | 100 times high that of β-rays |
| Effect on ZnS plate | cause luminescence | little effect | very little effect |
| Effect on photographic plate | affect photographic plate | cause much greater effect on photographic plate | very little or no effect |
Alpha beta and gamma rays Video
References
- Atkins, P. (2010). Shriver & Atkins’ Inorganic Chemistry (5th or later Edition). Oxford University Press.
- Lee, J. D. (2008). Concise Inorganic Chemistry: Fifth Edition by J.D. Lee (Fifth edition). Oxford University Press.
- Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012.






