Table of Contents
ToggleSingle electrode potential is the potential produced at the interface between solution and metal when the suitable metal is submerged in a solution containing its own ions. The half-cell reactions do not happen separately, therefore such potential cannot be determined.
Origin of single electrode potential
When a rod of metal say Zn is placed in a solution containing its own ion Zn++, then either of the following three possibilities arises.
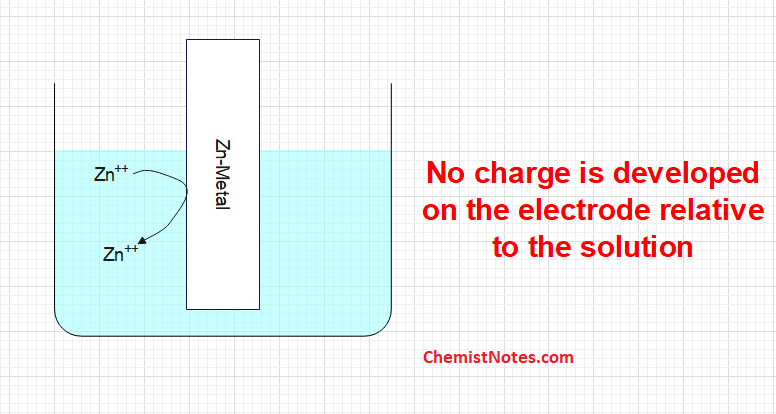
A.The Zn++ ion present in the solution may collide with the metallic rod and don’t undergo any change. In such case, no charge is developed on the electrode relative to the solution, such an electrode is known as a Null electrode.
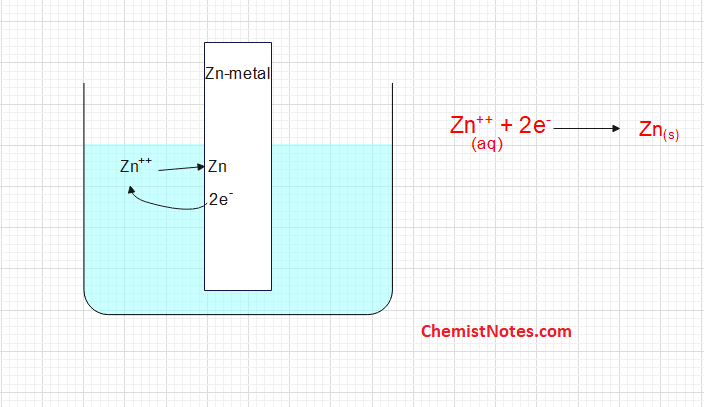
B.The Zn++ ion may collide with the metallic rod and get converted into metal atoms i.e ions are reduced.
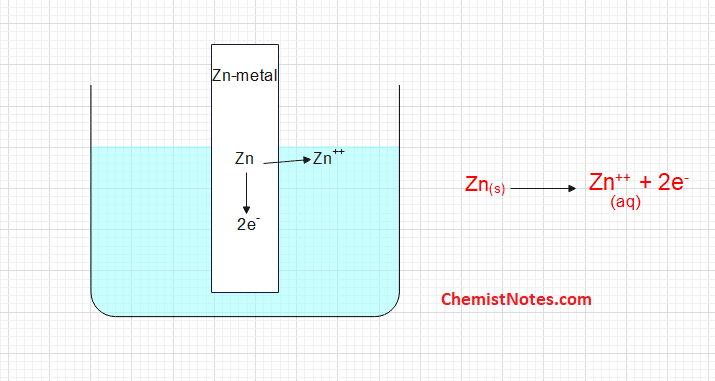
C. The metal atom on the electrode may lose electrons to the electrode and becomes Zn++ ion and go to the solution i.e oxidation occurs.
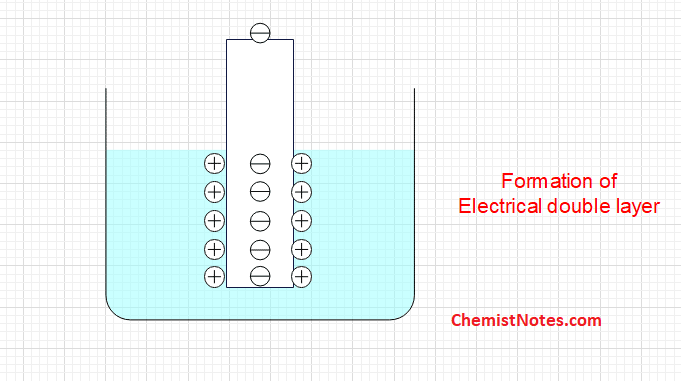
In this case, a negative charge is developed on the electrode due to the accumulation of electrons. The negative charge developed on the electrode doesn’t allow the metal atoms to continue losing electrons but it would re-attract the metal ions from the solution to neutralize its charge. Therefore, an electrical double layer is produced as shown.
Define single electrode potential
The electrical potential difference set up between the metal and the solution of its ions is known as single electrical potential or electrode potential. It is denoted by E and its value depends on the following factors:
- Nature of metal and its ion
- The concentration of ions in the solution
- Temperature.
We all know that a cell consists of two electrodes, oxidation takes place on one electrode and reduction takes place on another electrode. We can take the example of Daniel’s cell, where oxidation takes place at the zinc electrode and reduction takes place in the copper electrode. Each electrode is known as a half-cell.
The tendency of an electrode to lose or gain electrons i.e undergo oxidation or reduction when it is dipped in the solution containing its own ions is termed electrode potential or single electrode potential. Therefore, the electrode potential is the measure of the tendency of an electrode to undergo oxidation or reduction when it is in contact with the solution of its own ion.
As mentioned above, since there are two electrodes in the cell, electrode potential can be oxidation potential and reduction potential. Note that, the reduction potential of an electrode is the same as its oxidation potential with the opposite sign.
Standard electrode potential
Standard electrode potential can be defined as the electrode potential of an electrode when ions concentration is 1M, the temperature is 25 oC and at the pressure of 1 atmosphere if any gases are involved in the cell. It is denoted by Eo.
Measurement of Single electrode potential
We are not able to measure the single electrode potential of an isolated half-cell. We can only measure electrode potential difference produced when two half-cells are connected. In order to measure single electrode potential, it is necessary to couple an electrode with a reference or standard electrode.
Nernst equation for single electrode potential
The Nernst equation for single electrode potential can be expressed as:
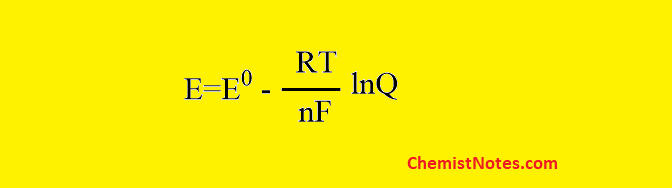
FAQs:
Can we measure the value of single electrode potential?
Unfortunately, there is no way to measure the value of the single electrode potential of an isolated half-cell.






