Table of Contents
ToggleLaws of photochemistry, mainly two laws Grotthus-Draper law and Einstein-Stork law of photochemistry are considered as basic laws of photochemistry. These laws have been discussed below.
Laws of photochemistry
First law of photochemistry
Grotthus Draper law is also known as first law of photochemistry. According to Grotthus Draper law, the radiation must be absorbed by a chemical substance in order for a photochemical reaction to occur.
In other word, First law states that,” only those radiations absorbed by a system can bring about a photochemical change”. It means the chemical substance must absorb radiation so that photochemical reaction takes place. Otherwise, without absorbing the light radiation any photochemical reaction can’t take place. Therefore, this law indicates the importance of light radiation in photochemical reaction.
It does not imply that radiation absorption must always be followed by a chemical reaction. When the conditions aren’t right for the molecules to react, the light energy goes to waste. It can be re-emitted in the form of heat or light.
The significance of this law is that photochemical reactions occur solely as a result of radiation absorption. This law provides no relationship between the amount of radiation absorbed and the number of moles of reactant undergoing reaction.
Second law of photochemistry
By applying the Quantum Theory of Light to photochemical processes, Stark and Einstein (1905) were able to study the quantitative component of the reactions.They discovered that each molecule in the reaction absorbs only one quantum of light, or photon. The molecule that gains one photon’s worth of energy becomes activated and begins a reaction. As a result, Stark and Einstein postulated a fundamental law of photochemistry, known as Stark-Einstein law of photochemical equivalence. This law is called Second law of photochemistry.
According to Stark-Einstein law, each molecule taking part in a photochemical reaction absorbs one quantum of radiation causing reaction to occur. In short, one molecule one quantum. In other words, a molecule before reacting photochemically must absorb one quantum energy.
Let a molecule AB of a chemical substance absorbs one photon and gets activated AB*, which gives product C.
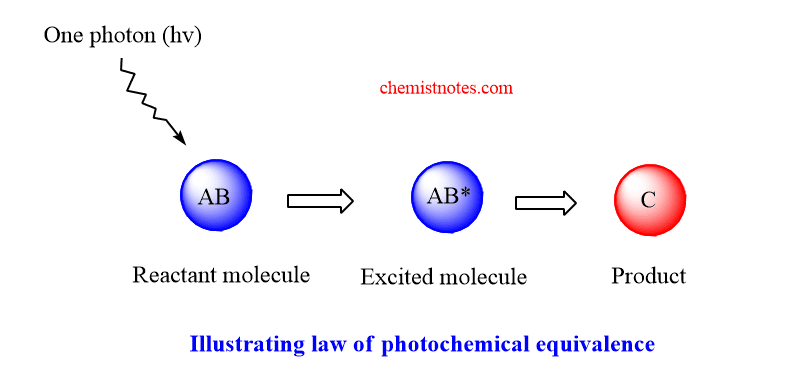
Generally we use in molarities, thus AB absorbs one mole of photons or one einstein of energy, E. The value of E is calculated.
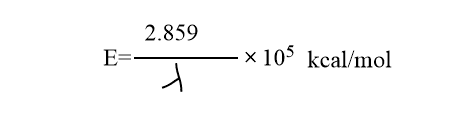
As a result, this law is concerned with the initial step of molecule activation.The number of molecules activated in the primary process is equal to the number of quanta absorbed. However, the molecules that are photochemically activated may sometimes initiate a series of chain reactions known as secondary processes. In this case, the number of reactant molecules converted to product is many orders of magnitude greater than the number of quanta absorbed, thus quantum yield value becomes high.
One Einstein
The quantity of energy E absorbed per mole of reacting substance is called one einstein.
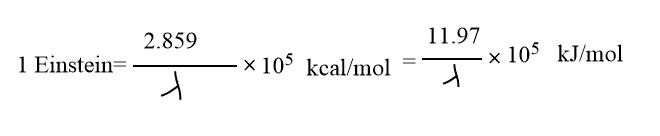
References
Arun Bahl, B. S. Bahl & G. D. Tuli, Essentials of Physical Chemistry, S. Chand and Company Ltd., New Delhi, 2012







2 Responses
Very useful and essential content
🙏🙏
Thank you very much.