Table of Contents
ToggleAuger electron spectroscopy (AES) is a technique used to identify the elemental composition and chemical bonding of the atoms in the surface region of the solid samples. It can be combined with ion-beam sputtering to remove the material from the surface and to continue to monitor the composition and chemistry of the remaining surface. It uses an electron beam as a probe of the sample surface and its output is the energy distribution of the secondary electrons released by the probe beam from the sample although only the Auger electron component of the secondaries is used in the analysis.
Principle of auger electron spectroscopy
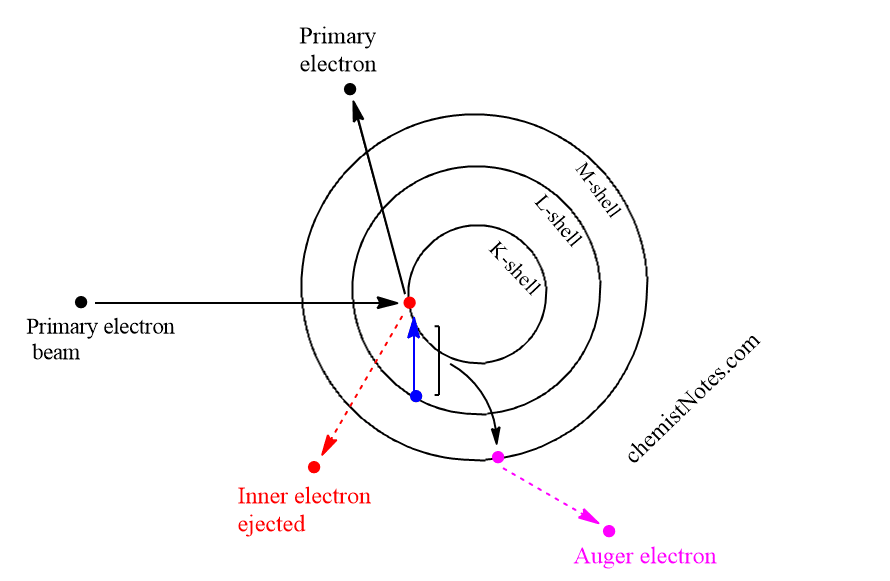
The basic Auger process involves the production of an atomic inner-shell vacancy usually by electron bombardment and the decay of the atom from this excited state by an electronic rearrangement and emission of an energetic electron rather than by emission of electromagnetic radiation.
If a surface is bombarded by a high-energy electron beam, some of the atoms of the surface will lose electrons from their K shell. The K shell vacancy will typically be filled by the decay of an electron from one of the L shells which leaves an energy excess equal to the difference between two shells K and L. This is sometimes relieved by the emission of characteristics X-ray, which is the basis for the EDS.
Most of the time, however, it is relieved by the ejection of another L-shell electron that overcomes its binding energy and carried off the remaining energy. This characteristic energy is the basis for the identification of the atoms in the sample. This electron is called Auger electron and the process is called Auger transition. Therefore, only the electrons that escape the sample with their characteristics Auger energy are useful in identifying the atoms in the sample.
Modes of AES
There are mainly three types of modes or methods of surface characterization using AES.
Point analysis mode
In this mode, the primary electron beam is positioned on the area of interest on the sample, and an Auger survey spectrum is taken.
Depth profiling mode
In this mode, an ion beam is directed to the same area that is being auger analyzed. The ion beam sputters materials off the surface so that analysis measures the variation in-depth of the composition of the new surface.
Auger mapping mode
Ln this mode, an auger peak of a particular element is monitored while the primary electron beam is raster-scanned over an area. This mode determines the spatial distribution across the surface.
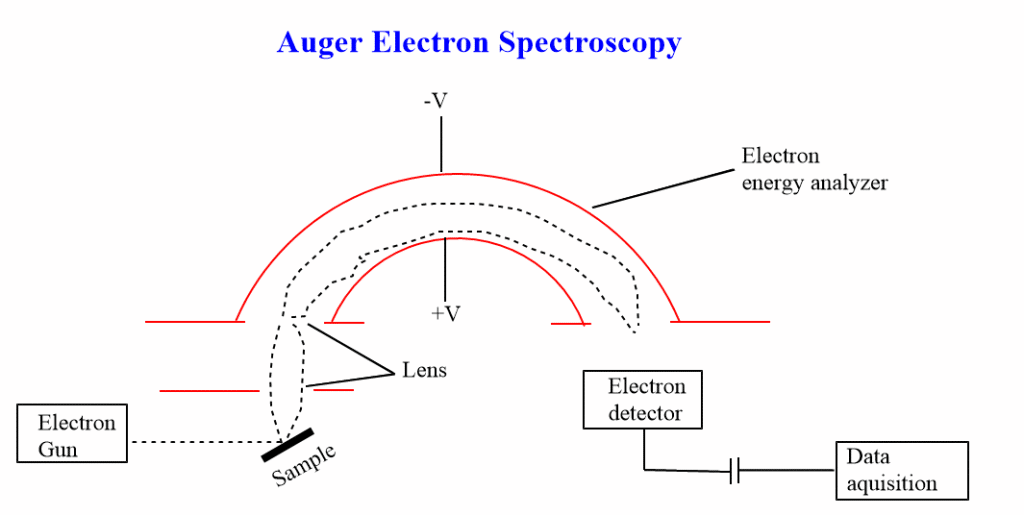
Auger electron spectroscopy instrumentation
Important elements of an auger spectrometer include:
- Vacuum system
- Electron source
- Electron energy analyzer
- Detector
- Recorder
Vacuum system
AES must be performed using an ultra-high vacuum system with pressure from 10-8 to 10-9 pa. Such a vacuum system is required to move absorbed gases from the sample, to eliminate the absorption of contaminants on the sample to increase the mean free path for electrons.
Electron source
Typical electron sources include tungsten filaments. In most cases, the electron source is thermionic but for the highest spatial resolution, the brighter field emission source may be used. The electron lens used to focus the beam may be either magnetic or electrostatic.
Electron energy analyzer
Concentric hemisphere analyzer (CHA) or cylindrical mirror analyzer (CMA) are used as analyzers.
Auger electron spectroscopy vs XPS
The main differences between the auger electron spectroscopy and x-ray photoelectron spectroscopy are listed below in the table.
| X-ray photoelectron spectroscopy (XPS) | Auger Electron Spectroscopy (AES) |
| XPS uses x-rays as a probe. | AES uses an electron beam as a probe. |
| It is a non-destruction technique | It is a destructive technique |
| It has a poor lateral spatial resolution | It has a good lateral spatial resolution |
| It has poor detectability | It has good detectability |
| It is not suitable for trace analysis | It is suitable for trace analysis. |
| All materials from biological to metal can be analyzed | A non-conducting sample can not be analyzed. |
Auger electron spectroscopy applications
The application of auger electron spectroscopy is discussed under the following points.
- Qualitative information: Which element is present within the sampling volume of the measurement.
- Quantitative information: This tells how much of each element is present.
- Chemical information: This shows the chemical state in which these elements are present.
- It gives the electronic structure of material such as the valence band density of states.
Advantages and disadvantages of auger electron spectroscopy
Advantages of AES
- It has a good lateral spatial resolution as low as 300Å
- It has very good depth resolution as low as 20 Å
- It has good absolute detectability as low as 100 ppm hence used for trace analysis.
- It can produce a three-dimensional map of the composition and chemistry of a volume of a sample.
Disadvantages of AES
- It is a three-electron process, elements with less than three electrons can not be analyzed. Therefore, hydrogen and helium can not be detected.
- It is destructive in nature because an electron beam as a probe can be destructive to some samples.
- Some non-conducting samples charge under-electron beam probing and can not be analyzed.
Auger electron spectroscopy youtube video
References
C. R. Brundle, C. K. Evans, Jr., S. Wilson and L. E. Fitzpatrick (eds), Encyclopedia of Materials Characterization: Surfaces, Interfaces, Thin Films, Butxetworch-Heinemann, Reed Publishing (USA) Inc., 1992






