Table of Contents
ToggleSuzuki reaction is a palladium-catalyzed cross-coupling reaction between aryl halide or vinyl halides and organoboron compounds such as boronic acid or boronic ester. This reaction is also known as the Suzuki-Miyaura reaction because it was first reported by Suzuki and Miyaura in 1979. This reaction can tolerate many types of functional groups and yields are usually very good. Thus, the reaction has gained increased importance in recent years in synthetic organic chemistry.
Suzuki reaction
The organoboron compound is reacted with aryl or vinyl halide in presence of palladium catalyst to form a carbon-carbon bond. This reaction is known as the Suzuki reaction or Suzuki coupling reaction.
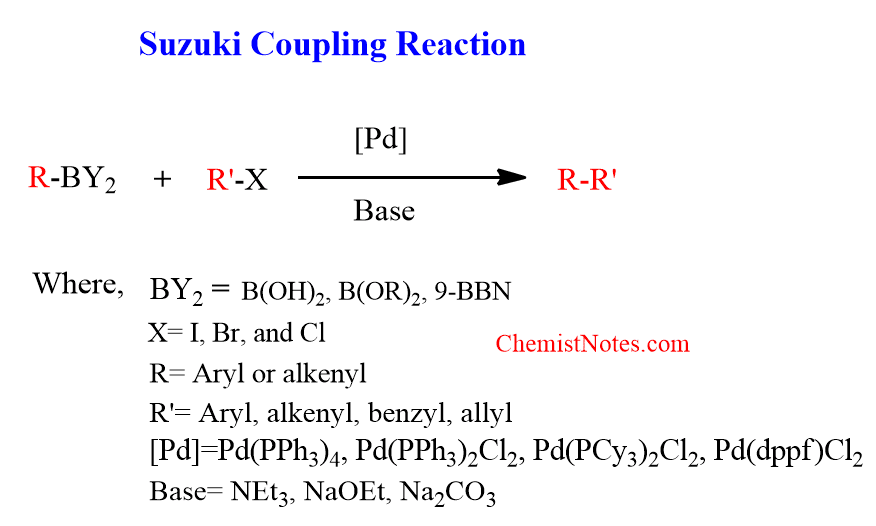
Moreover, the mostly used palladium catalysts are Pd(PPh3)4, Pd(PPh3)2Cl2, Pd(PCy3)2Cl2, Pd(dppf)Cl2. In contrast to Heck’s reaction, Suzuki’s reaction does not work under neutral conditions but the conditions are milder than that of heck’s reaction. This reaction requires a suitable base such as sodium hydroxide, or sodium/potassium carbonate in the transmetallation step of the catalytic cycle.
What are the functions of the base in the Suzuki reaction?
- Base activates the catalyst for transmetallation.
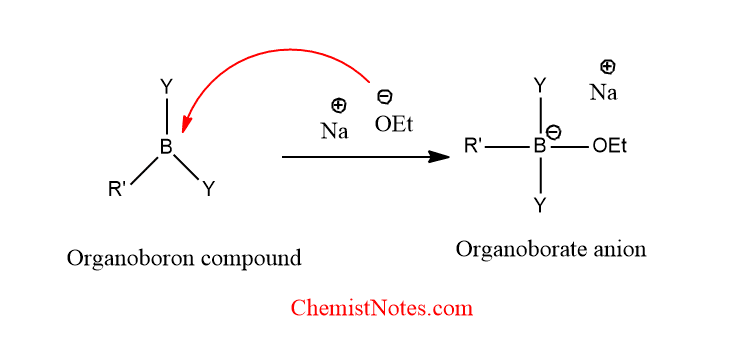
- It also activates the organoboron compound by converting it into the organoborate anion, thus making the transmetallation process rapid.
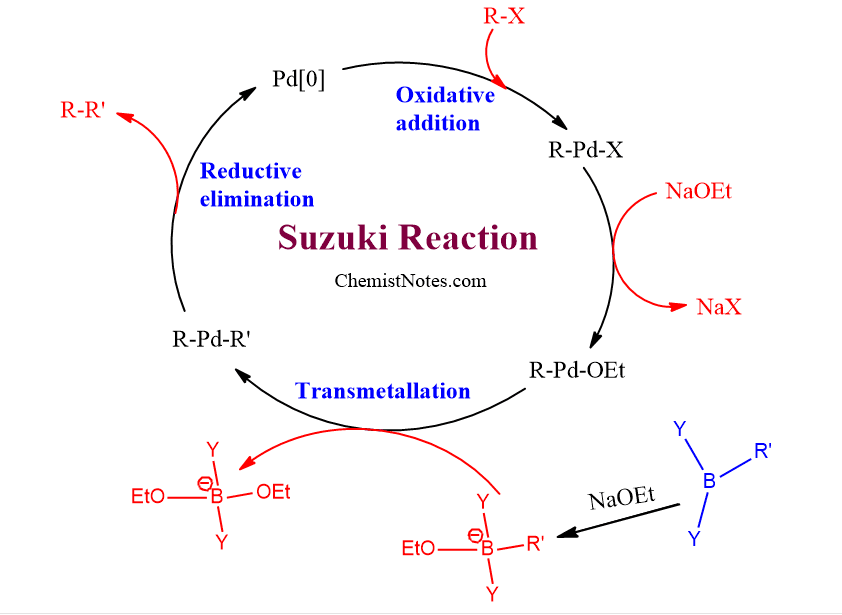
Suzuki coupling reaction mechanism
The mechanism of the Suzuki reaction involves the catalytic cycle, which includes three steps: Oxidative addition, Transmetallation, and Reductive elimination.
- Oxidative addition: The active catalyst in this reaction is Pd[0] and the aryl or vinyl halide reacts with it to form Pd[II]. Here, the oxidation state of Pd changes from zero to 2, and the halide component is added to the Pd center. Thus, this step is termed Oxidative addition.
- Transmetallation: In this step, the transfer of the R’ group from the boron to the Palladium center takes place. Here, R’ group changes its center from Boron to Palladium. Thus, this step is called transmetallation, which means ligand transfer from one metallic center to another one.
- Reductive elimination: In this step, the coupled product(R-R’) is formed and a catalytically active Pd[0]-complex is generated. Here, the oxidation state of Pd changes from Pd[II] to Pd[0], and the target product is eliminated simultaneously.
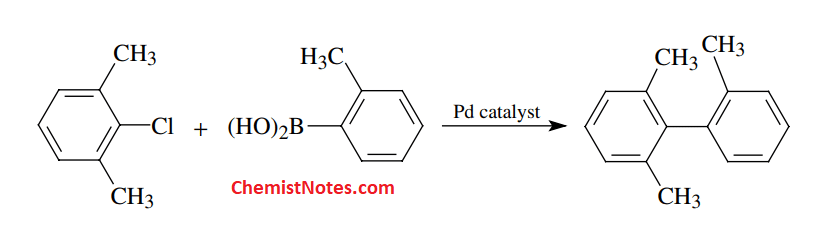
Application of Suzuki coupling reaction
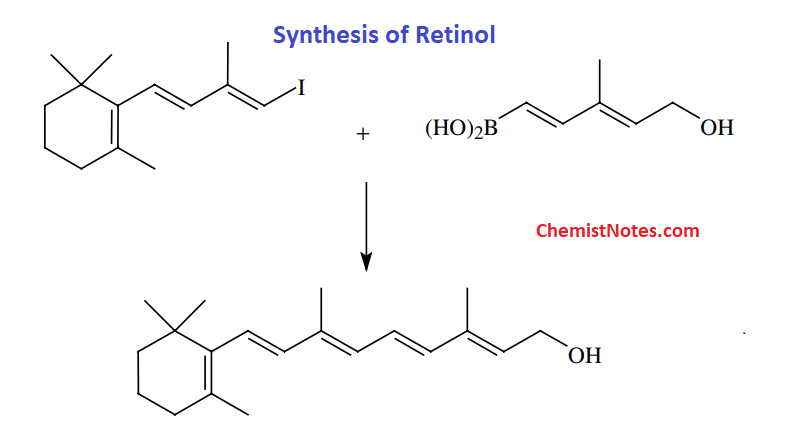
The pharmaceutical industry mainly uses this reaction for the production of useful medicine precursors since boron species formed as a side product are relatively non-toxic. Moreover, This reaction is often used to make substituted biphenyls, poly-olefins, and styrenes as well as retinol.
Synthesis of Retinol
Synthesis of Biphenyls