Table of Contents
ToggleIn simple distillation, the liquids mixture is simply heated to boiling points and condensing back them to liquids.
Condition:
- This methods is only effective if the liquid of mixture has sufficiently different boiling temperature i.e at least 25 degree Celcius difference in boiling points.
- If the liquid contains nonvolatile impurities, then it can be a best method to purify.
Disadvantages of Simple Distillation
The liquid mixture having similar boiling points can’t be separated because both evaporate at the same temperature and both are converted to vapor and we can’t collect separately.
Process of Simple distillation
During distillation, the mixture of liquids having sufficiently different boiling points is heated in round bottom flask fitted with a condenser. The liquid having the lowest boiling points evaporates first, and which passes through condenser and is converted back to liquids form which is collected in the pure form called distillate. The remaining mixture is heated further and if sufficient temperature is reached, the remaining liquid is converted to vapor and which is also collected by condensing. In this way, distillation has an important role in the purification of liquids mixture
Experimental set up/Apparatus of simple distillation:
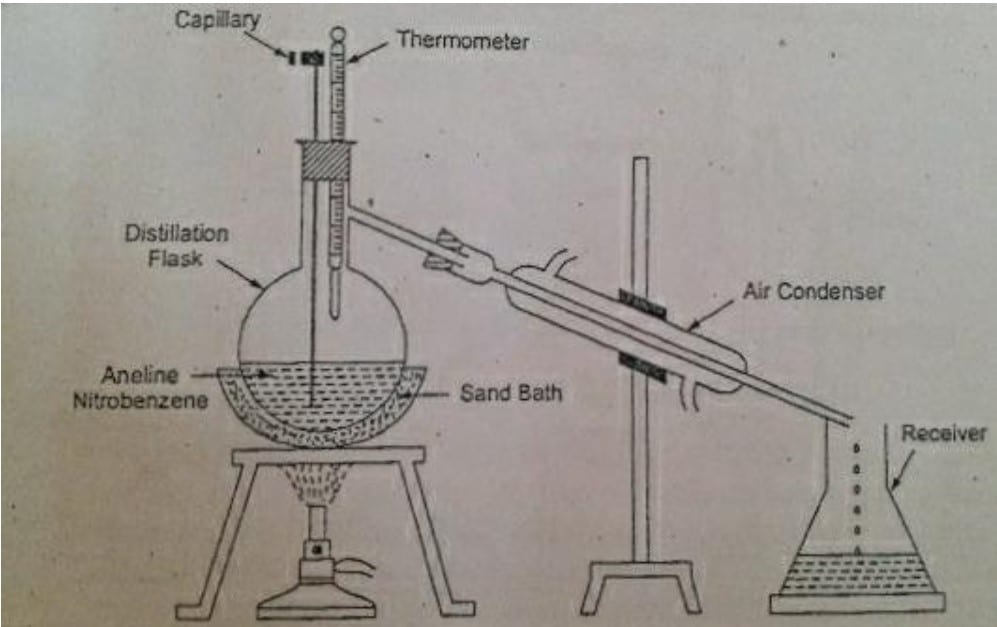
Example of Simple distillation: Separation of aniline and nitrobenzene from the mixture by simple distillation.
The experimental setup is shown in above figure. The mixture of aniline and nitrobenzene is taken in a distillation flask fitted with an air condenser, and a thermometer. A very thin capillary is immersed in the mixture to be distilled to avoid bumping of liquids and the upper end is closed by a clamp.
The boiling point of aniline is 183 degree Celcius and that of nitrobenzene is 211degree Celcius. The liquid mixture is heated over the sand bath and when the temperature reaches near the boiling point of aniline, it starts to vaporize. The temperature is maintained at 183 degree Celcius and vapors are collected in the receiver by condensing.
When the aniline is completely distilled, a new receiver is used and the temperature of system is increased to 211 degree Celcius. Now, the nitrobenzene starts distilling and vapors condense while passing through the air condenser and liquid nitrobenzene is collected in the receiver.
What is simple distillation?
Simple distillation is a type of distillation in which the liquids mixture is simply heated to boiling points and condensing back them to liquids.
When is simple distillation used?
Simple distillation is used when






