Table of Contents
ToggleNitrene Definition: Nitrenes are electron deficient and reactive intermediates, in which the structure of nitrene reveals that there are six electrons around nitrogen. These are nitrogen analogs of carbene and hence something referred to as azo carbene.
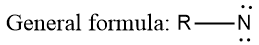
Nitrene Hybridization
Nitrenes are nitrogen analogues of carbenes. The nitrogen atom possesses only six electrons; in nitrenes, the triplet state is lower in energy than the singlet state.
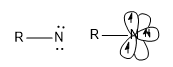
Nitrene Examples
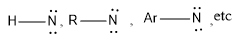
Singlet and triplet nitrenes
Singlet nitrene is a kind of nitrene in which unshared electrons are paired. It is represented as:
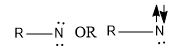
Triplet nitrene is a nitrene in which unshared electrons are not paired. It is represented as:
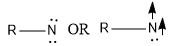
Generation/ Formation of Nitrene
- By the reduction of nitro compound with trialkoxy phosphite
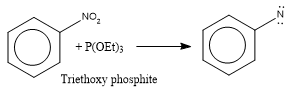
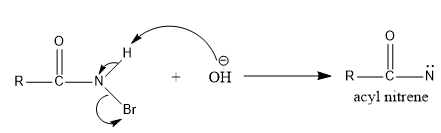
- By α-elimination reaction
Reactions of Nitrene
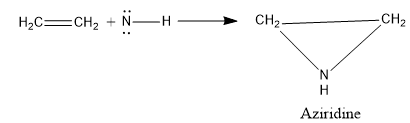
- Addition to C=C double bond
- Rearrangement reaction
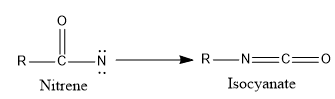
- Dimerization
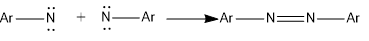
Carbene and Nitrene
Carbenes are very short-lived species or reactive species in which the carbon atom has two bond pairs and two unshared electrons.
FAQs/Mcqs
What is nitrene?
Nitrenes are electron deficient and reactive intermediates, in which the structure of nitrene reveals that there are six electrons around nitrogen.






