Table of Contents
ToggleMemory Effect stands for the condition despite the fact that the two reaction routes share a seemingly identical intermediate carbocation, the solvolysis of two diastereomers might result in the same two products but in different ratios. In other words, those chemical reactions which are proceed through formation of carbocation intermediate, initials carbocation seem to be remembering as its origin, but intermediated undergoes further evolution through different reaction mechanism. In certain reactions, the carbocations appear to recall how they first came into being and often respond in the same way going forward. These are referred to as memory effects.
Each mechanism gives an identical product but in different ratio. For example, the solvolysis of a bicyclic compound from its Exo and endo isomers give exactly the same carbocation, and progression reaction of an intermediate follow pathways with different mechanism.
Example of memory effect
Solvolysis of endo and Exo isomers of norborn-2-en-7-ylmethyl-X:
The solvolysis mechanism, two endo isomer and Exo isomers of the compound norborn-2-en-7-ylmethyl-X is unveiled below:
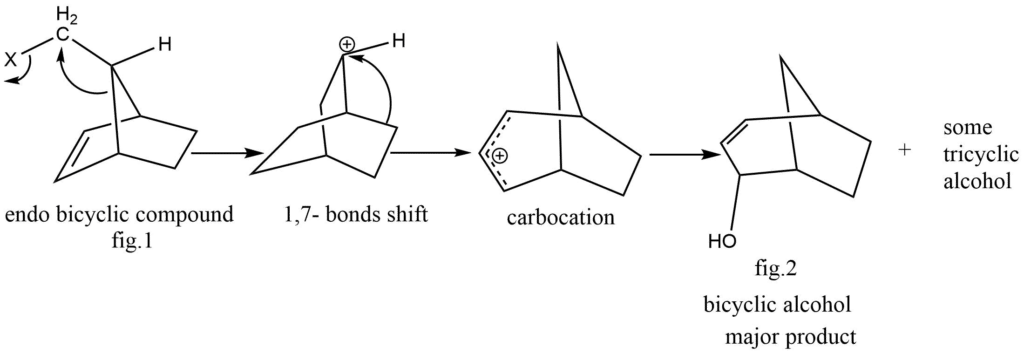
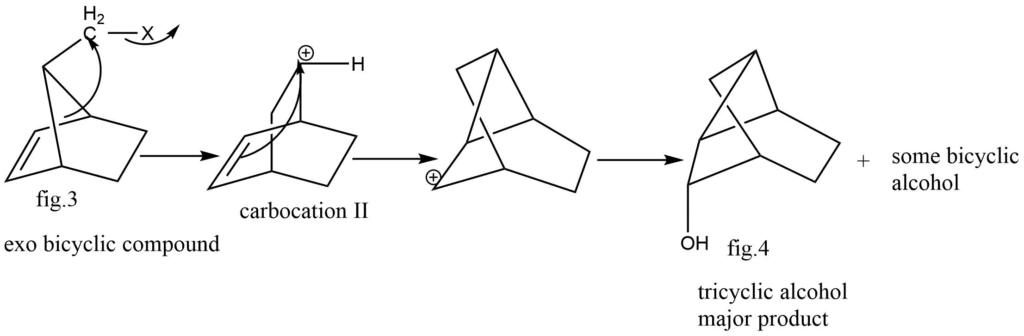
Bicyclic alcohol is the main product of the solvolysis of endo isomer, minor quantity of tricyclic alcohol is also formed. While an exo isomer subjected in a solvolysis tricyclic alcohol (fig.4) is produced as a main product also a minor quantity of bicyclic product is formed, the two cases’ solvolysis mechanisms followed the same carbocation intermediate pathway, while the subsequent reactions of the two intermediates were different.
Carbocation(I), the exo isomer’s intermediate in the reaction, undergoes intramolecular addition, whereas carbocation(II), the endo isomer’s intermediate, undergoes 1,7-bond shift. In twisted I, the carbocation is far away from the π electrons of double bond and cannot perform intramolecular addition. Contrarily, the twisted II form geometry exhibits intermolecular addition rather than bond shifting because of the closed seeming between-carbocation π electrons of double bond system.
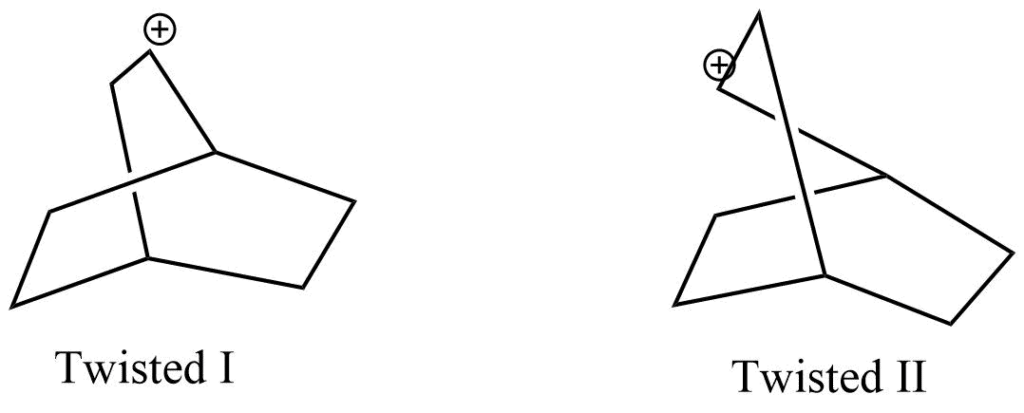
The actual cause of the memory effect hasn’t been identified yet. However, some possible causes are:
- Two carbocations have different salvation affinities.
- Geometrical difference between their twist forms.
- Ion pairing may be responsible.
- Involvement of non-classical carbocation
MCQs/FAQs
What is memory effect in molecular rearrangement?
A beginning carbocation’s geometrical characteristics influence the competitive interaction between an empty p orbital and a orbital or a p/ orbital, which determines the preference for the rearrangement to G or L (the memory effect).






