Table of Contents
ToggleChromatographic plate theory
Chromatographic plate theory assumes that the chromatographic column has a high number of distinct layers, referred to as theoretical plates. All chromatographic separations are based on the difference in the extent to which solutes are partitioned/distributed between the stationary phase and mobile phase. For the equilibrium distribution of the solute between two phases, the partition/distribution coefficient, K is given by

Chromatographic column:
According to Martin & Synge, a chromatographic column consists of a series of discrete yet continuous horizontal layers called the theoretical plates. Separate equilibrations of the sample between stationary and mobile phases occur in these plates. The analyte moves down the column by transfer of equilibrated mobile phase from one plate to the next.
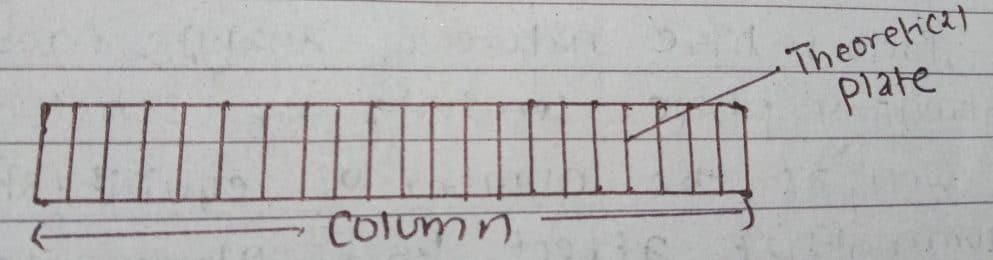
It is used as a means for expressing the efficiency of a chromatographic system. As the number of theoretical plates increases, the efficiency of separation in a chromatographic column also increases because of the increase in equilibrations number. The theory assumes that the solute during its passage through the column is always in equilibrium with the mobile and stationary phase. The size of the cell is chosen to provide sufficient residence time for the solute to establish equilibria with the two phases. Thus, the smaller the plate, the faster the equilibrium will be, and the more the plates there will be in the column. The plate theory accounts for the Gaussian shape of chromatographic peaks and their rate of movement down a column but fails to account for peak broadening.
Differences in the value of partition coefficient (k), four different analytes result in them separating into distinct zones of bands. There are several factors like the nature of the analyte, mobile and stationary phase, sensitivity of the detector as well as the number of equilibrations in the column that affect the chromatographic separation. It is important to note down that the theoretical plates are hypothetical, and are a way of describing the analyte movement through the column.
If the length of the column is ‘L’ and the height equivalent of the theoretical plate is H, then the number of theoretical layers ‘N’ is given by
N = L/H
L = NH
The HETP, Height equivalent of the theoretical plate is defined as the height of a layer of the column such that the solution leaving the layer is in equilibrium with the average concentration of the solute in the stationary phase throughout the layer. The Less the HETP or ‘H’ value, the more efficient the separation.
Limitations of plate theory
- The plates are symmetrical as it assumes that k is linear.
- The thickness of the phases is not being considered.
- Rapid equilibrations of analyte between the mobile phase and stationary phase.






