Table of Contents
ToggleAcid base catalysis involves the study of those reactions catalyzed by either acid or base. Many reactions are catalyzed by acids or bases, and some are catalyzed by both acids and bases. For example, the ester can be hydrolyzed by both acid and base.
What is acid base catalysis?
Some acids as well as bases act as catalysts for the reaction. Therefore, acid base catalysis can be defined as the process in which the reaction is catalyzed by acids or bases.
Acid or base can catalyze the reaction in two ways:
- General acid base catalysis
- Specific acid base catalysis
General acid base catalysis
This catalysis can be further categorized as general acid catalysis and general base catalysis. Let’s discuss them with suitable examples and mechanisms.
General acid catalysis
Some reactions are known which are catalyzed not only by H3O+ but by other acids in the system as well. This is known as general acid catalysis. The “general” means the catalysis is by proton donors in general and not by H3O+ alone.
In other words, if the reaction rate is increased not only by an increase in lyonium ion [SH+] concentration but also by an increase in the concentration of other acids. This is known as general acid catalysis. These other acids increase the rate even when [SH+] is kept constant.
The relationship between the acid strength of the catalyst and its catalytic ability can be expressed by the Bronsted catalysis equation as:
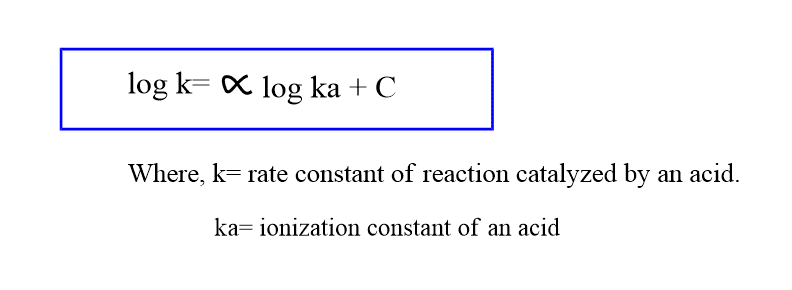
A common example of such a type of catalysis is the hydrolysis of ortho esters.
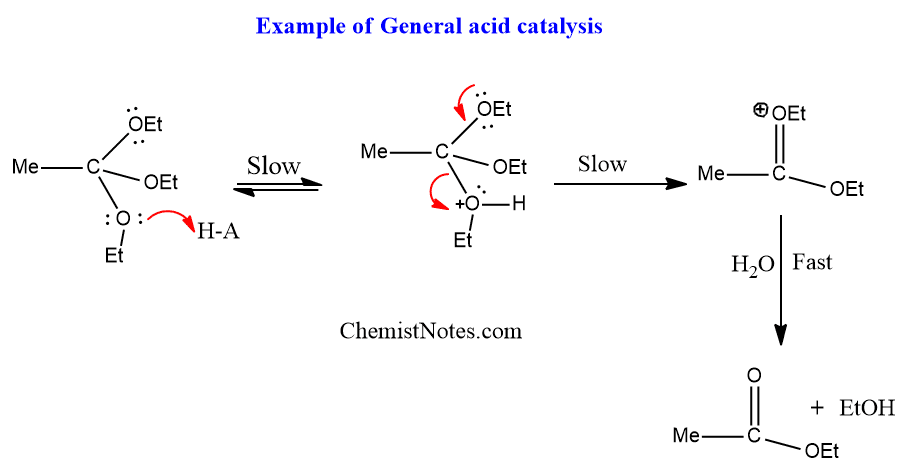
General acid catalysis is characteristic of those reactions in which protonation of the substrate is slow i.e rate determining step and is followed by the rapid conversion of the intermediate into products.
General base catalysis
In general base catalysis, bases other than OH– are involved. In this reaction, the rate of reaction is increased not only by an increase in [S–] concentration i.e OH– concentration but also by an increase in the concentration of other bases.
The relationship between the base strength of the catalyst and its catalytic ability can be expressed by the Bronsted catalysis equation as:
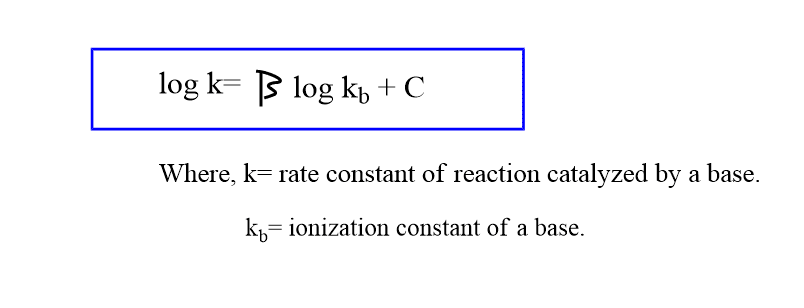
A common example of such a type of catalysis is the bromination of acetone in an acetate buffer.
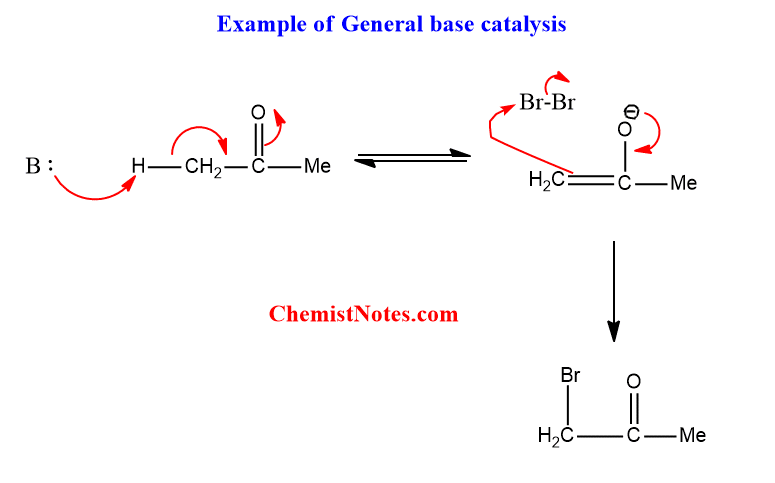
Specific acid base catalysis
Specific means the reaction is either catalyzed only by [H3O+] or by [OH]–, not by other acids or other bases. This catalysis can be further categorized as specific acid and specific base catalysis. Let’s discuss them with suitable examples and mechanisms.
Specific acid catalysis
Reactions are known which are catalyzed by only [H+] i.e [H3O+] in aqueous media but not by other acids in the system. This is known as specific acid catalysis, specific because the catalysis is by only H3O+.
A common example is the hydrolysis of simple acetals.
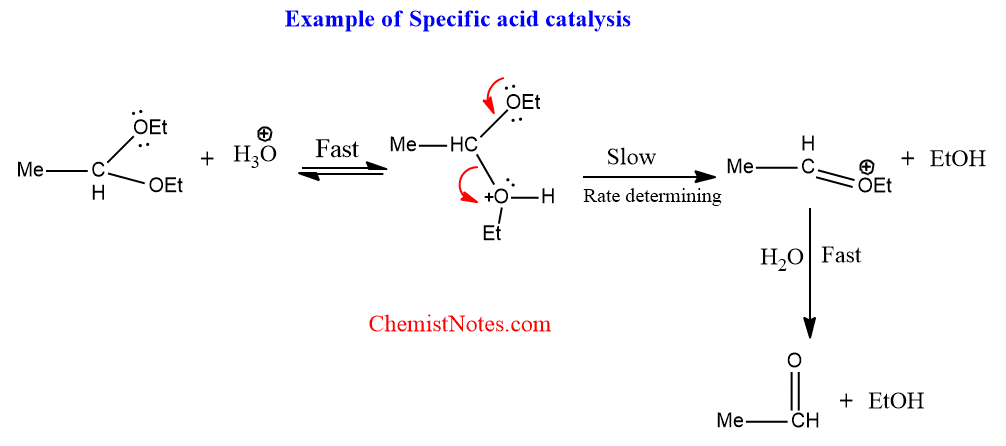
Specific acid catalysis is characteristic of reactions in which there is rapid, reversible protonation of the substrate before the slow, rate-determining step.
Specific base catalysis
In this catalysis, the rate of reaction is increased by an increase in [S–] i.e OH– only but not only other base.
A common example of such a type of catalysis is the reversal of aldol condensation.
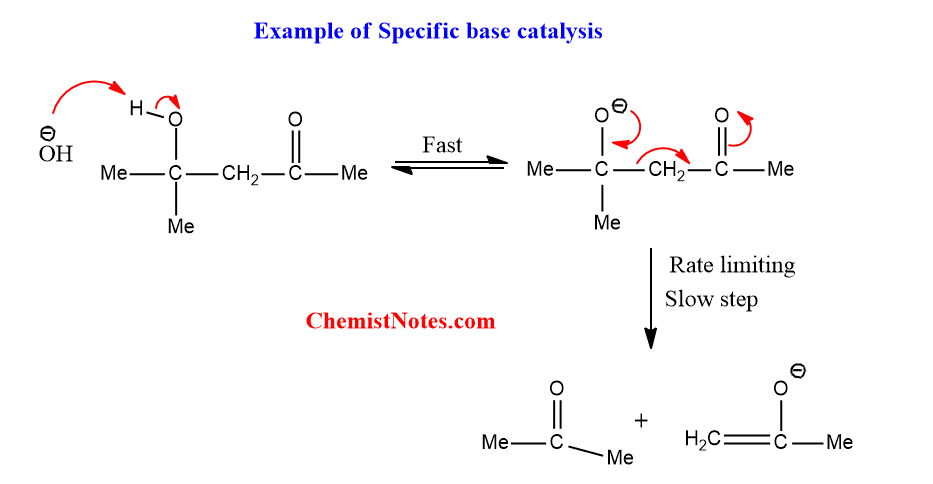
Specific base catalysis is characteristic of reactions in which there is rapid, reversible proton removal from the substrate before the slow, rate-limiting step.
Acid-Base catalysis video
References:
- Skyes, P., A Guide Book to Mechanism in Organic Chemistry, Second edition, Orient Longman Ltd., 1988






