Table of Contents
ToggleIn the plant kingdom, phenolic compounds are widely distributed and makeup one of the most important subgroups of secondary metabolites. They can be categorized as simple phenols or polyphenols based on how many phenol units are present in the molecule and have at least one aromatic ring bearing one or more hydroxyl groups. The importance of phenolic compounds is enormous. They constitute a broad family of bioactive secondary metabolites.
Phenolic compound definition
Phenolic compounds are the secondary metabolites in different fruits, and cereals that contribute to antioxidant activity. Generally found in fruits, vegetables, olives, grains, coffee beans, and tea leaves. It has at least one aromatic ring with one or more hydroxyl groups. Depending on how many phenol units are present in the molecule, it can be categorized as either a simple phenol or a polyphenol. Numerous biological functions have been assigned to them, with the antioxidant function being the most significant.
Additionally, secondary metabolites serve as signaling molecules that draw animals or pollinators for seed dispersal while also shielding the plant from oxidants and UV radiation.
Phytochemicals
In general, phytochemicals are chemical substances that plants create to aid in their ability to fend off illnesses from fungi, bacteria, and plant viruses. They are also substances that insects and other animals eat. The name derives from the Greek word “plant,” and “v” (phyton). The principal defense against oxidative stress, the root of most chronic diseases, is provided by phytochemicals, which are bioactive, non-nutrient substances.
Source of phenolic compound
According to those studies, the main dietary sources of phenolic compounds include fruits, vegetables, and drinks. Plant polyphenols may offer dietary antioxidant protection for human health and disease.
Particularly high in phenolic compounds are cocoa, potato, yam, tomato, kale, Brussels sprouts, broccoli, and other dark green leafy and colorful vegetables, as well as legumes, cereals, spices, and fruits like cherries and citrus.
Classification of phenolic compound
Based on chemical structures, phenolic compounds are divided into the following subclasses:
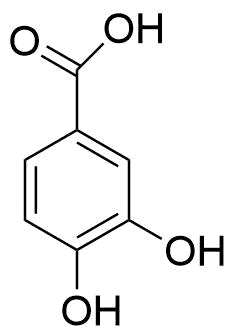
1. Phenolic acid
The chemical structure of phenolic acids includes at least one aromatic ring with at least one hydrogen replaced by a hydroxyl group. They are made up of two groups: hydroxybenzoic acids (HBAs) and hydroxycinnamic acids (HCAs), which are made from nonphenolic benzoic and cinnamic acid molecules, respectively. The precursory ingredient for their synthesis is l-phenylalanine or l-tyrosine, which is done via the shikimate pathway.
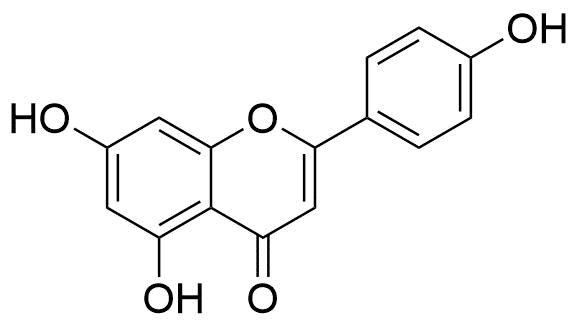
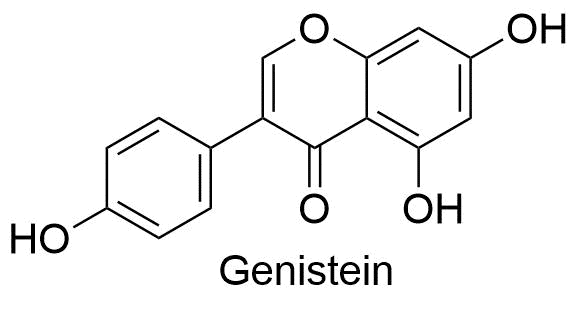
2. Flavanoids
The basic building blocks of flavonoid structures are two aromatic rings, A and B, connected by a three-carbon bridge, which is typically a heterocyclic ring, C. The acetate/malonate pathway, where ring A is produced, and the shikimate pathway, where ring B is derived from phenylalanine, are the sources of flavonoid molecules.
The primary flavonoid groups, including flavonols, flavones, flavanones, flavanols (catechins), isoflavones, flavanonols, and anthocyanidins, are all produced by differences in the substitution patterns of C rings. The many chemicals within the flavonoids class are produced by variations in the A and B rings.
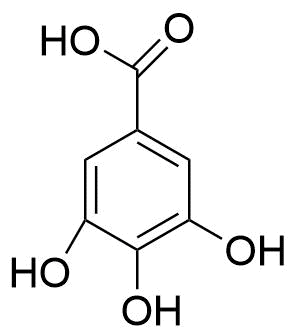
3. Tannins
Tannins, also known as tannic acid, play an important role in how plants and their ecosystems interact. For instance, they can act as antimicrobial agents or as a deterrent to herbivores. These compounds contain a large number of hydroxyl or other functional groups and therefore are found in the form of esters or heterosis.
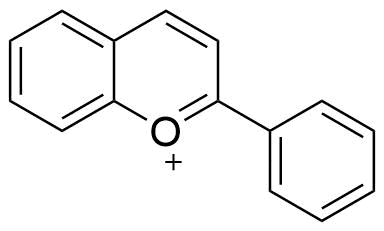
4. Coumarins
The chemical compound coumarin, also known as 2H-chromen-2-one, has the formula C9H6O2. In order to form a second six-membered heterocycle that shares two carbons with the benzene ring, its molecule can be compared to benzene with two neighboring hydrogen atoms swapped out for a lactone-like chain.
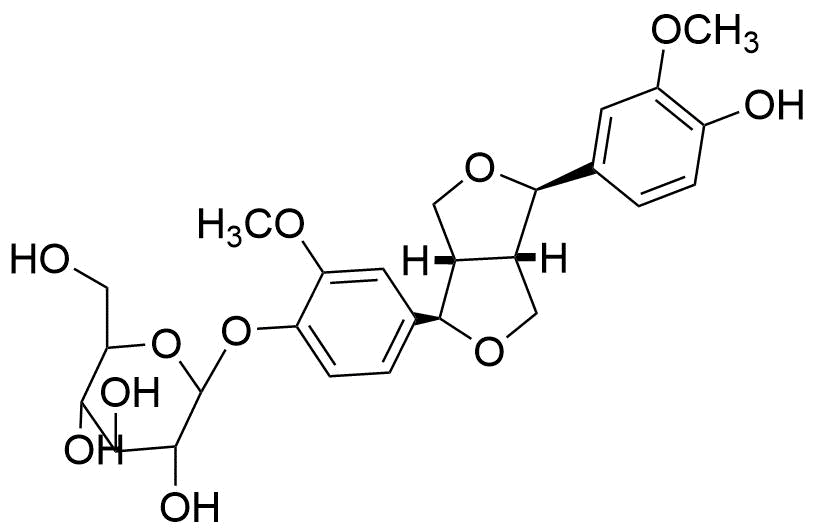
5. Lignans
Lignans are polyphenolic substances that are present in plants. (Additional details) Many different plant-based foods, such as seeds, whole grains, legumes, fruit, and vegetables, contain lignan precursors.
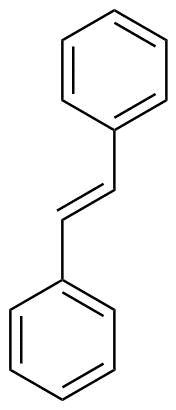
6. Stilbenes
The broad class of plant secondary metabolites known as stilbenes belongs to the polyphenol family. They can be found in a variety of food (such as grapes, peanuts, and other foods) and medicinal plants, such as Polygonum multiflorum, Polygonum cuspidatum (Polygonaceae), Hopea chinensis (Dipterocarpaceae), Gnetum parvifolium (Gnetaceae), Caragana sinica (Leguminosae), and Morus alba (Moraceae).
Natural stilbenes, which primarily consist of stilbene monomers, polymers, and heteromers, are polyhydroxy phenolic substances generated when hydroxyl groups replace hydrogen atoms in various benzene ring places.
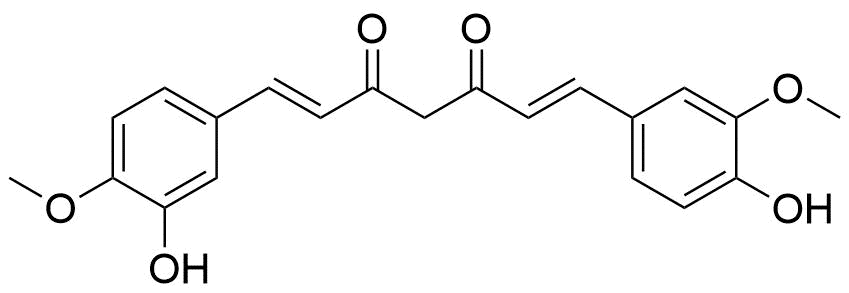
7. Curcuminoids
The active components of the dietary spice turmeric, curcuminoids (particularly curcumin), are derived from the rhizomes of Curcuma longa, a plant that belongs to the ginger family and has been used medicinally for many years, primarily in Asian countries.
Method for the determination of Phenolic compound in Samples
The method for the determination of total phenolic compounds is discussed below:
Folin-Ciocalteu Technique
Using the Folin Ciocalteu technique, the total phenolic content was calculated as the gallic acid equivalent (GAE) in mg/g of the extract. Under UV light and throughout the day, the fluorescence properties of the powdered drugs and subsequent extracts with and without chemical treatment were noted. The whole process is already described in our previous post.
You can easily go through it. Determination of Total Phenolic Content
Application of phenolic compounds
There is a vast spectrum of biological activity that phenolic compounds exhibit. For instance, they have been found to have anti-inflammatory, antibacterial, and antioxidant activities. They are common in the natural world.
Curcumin may aid in the treatment of oxidative and inflammatory diseases, metabolic syndrome, arthritis, anxiety, and hyperlipidemia, according to research. Additionally, it might aid in the control of inflammation and muscle soreness brought on by exercise, improving recovery and subsequent performance in physically active people.
Stilbene is used to make dyes, optical brighteners, phosphors (which emit light), and scintillators.
References
https://doi.org/10.1016/B978-0-12-814774-0.00002-5
Pubmed, ChemSpider, Wikipedia